Enasidenib
- CAS NO.:1446502-11-9
- Empirical Formula: C19H17F6N7O
- Molecular Weight: 473.38
- MDL number: MFCD29472245
- SAFETY DATA SHEET (SDS)
- Update Date: 2024-11-19 20:33:22
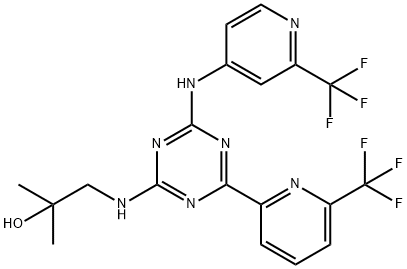
What is Enasidenib?
Absorption
Following a single oral dose of 100mg enasidenib, the peak plasma concentration of 1.3 mcg/mL is reached at 4 hours after ingestion. The absolute bioavailability is aproximately 57% and the steady-state plasma levels are reached within 29 days of once-daily dosing .
Toxicity
Enasidenib is not clastogenic or mutagenic in vitro. In vitro repeat-dose toxicity studies, enasidenib treatment induced changes in male and female reproductive systems including seminiferous tubular degeneration, hypospermia, atrophy of the seminal vesicle and prostate, decreased corpora lutea and increased atretic follicles in the ovaries, and atrophy in the uterus .
Description
Enasidenib (1446502-11-9) is a potent (IC50’s = 100 nM IDH2R140Q homodimer, 30 nM IDH2R140Q/WT heterodimer and 10 nM IDH2R172K/WT heterodimer) and selective inhibitor of mutant isocitrate dehydrogenase 2 (IDH2).1? It suppressed the production of the oncometabolite (R)-2-Hydroxyglutarate (a competitive inhibitor of αKG-dependent dioxygenases which leads to epigenetic dysregulation) and induced cellular differentiation in primary human IDH2 mutation-positive acute myeloid leukemia cells.1,2? Recently approved for clinical use by the FDA.
The Uses of Enasidenib
Enasidenib is a first-in-class oral selective inhibitor of mutant IDH2 enzymes (isocitrate dehydrogenase 2), for the treatment of adults with relapsed or refractory IDH2-mutated acute myeloid leukemia.
Indications
Indicated for the treatment of adult patients with relapsed or refractory acute myeloid leukemia (AML) with an isocitrate dehydrogenase-2 (IDH2) mutation.
Background
Enasidenib is an orally available treatment for the treatment of adult patients with relapsed or refractory acute myeloid leukemia (AML) with specific mutations in the isocitrate dehydrogenase 2 (IDH2) gene, which is a recurrent mutation detected in 12-20% of adult patients with AML . Patients eligible for this treatment are selected by testing the presence of IDH2 mutations in the blood or bone marrow. This small molecule acts as an allosteric inhibitor of mutant IDH2 enzyme to prevent cell growth, and it also has shown to block several other enzymes that play a role in abnormal cell differentiation. First developed by Agios Pharmaceuticals and licensed to Celgene, enasidenib was approved by U.S. Food and Drug Administration on August 1, 2017.
Definition
ChEBI: Enasidenib is a 1,3,5-triazine which is substituted by (2-hydroxy-2-methylpropyl)nitrilo, 6-(trifluoromethyl)pyridin-2-yl and [2-(trifluoromethyl)pyridin-4-yl]nitrilo groups at positions 2,4 and 6, respectively. It is an isocitrate dehydrogenase-2 (IDH2) inhibitor which has been approved for the treatment of adults with relapsed or refractory acute myeloid leukaemia (AML). It has a role as an antineoplastic agent and an EC 1.1.1.42 (isocitrate dehydrogenase) inhibitor. It is an aminopyridine, an organofluorine compound, a secondary amino compound, a tertiary alcohol, a member of 1,3,5-triazines and an aromatic amine.
Pharmacokinetics
In a study involving adult patients with relapsed or refractory AML, overall response rate of 40.3% was achieved in enasidenib therapy which was associated with cellular differentiation and maturation, typically without evidence of aplasia . Enasidenib is not shown to cause QTc prolongation.
in vitro
ag-221 was found to be able to reduce 2-hg levels by >90%, reverse in-vitro histone and dna hypermethylation, and induce differentiation in leukemia cell model as well. in addition, a dose dependent proliferative burst of the human specific cd45+ blast cells was observed by the treatment of ag-221, as measured by the expression of cd11b, cd14, cd15 and cell morphology [1].
in vivo
the efficacy of ag-221 in a primary human aml xenograft model with the idh2 r140q mutation was studied, and the results showed that ag-221 could reduce 2-hg in the plasma, bone marrow, and urine of engrafted mice potently. in addition, the treatment of ag-221 could also induce a significant and dose dependent survival benefit as demonstrated by that all mice in the high dose treatment of ag-221 survived to the end of study [1].
Metabolism
Enasidenib undergoes N-dealkylation mediated by multiple CYP (CYP1A2, CYP2B6, CYP2C8, CYP2C9, CYP2C19, CYP2D6, and CYP3A4) and UGT (UGT1A1, UGT1A3, UGT1A4, UGT1A9, UGT2B7, and UGT2B15) enzymes to form AGI-16903, as suggested by in vitro studies. AGI-16903 can be further metabolized by CYP1A2, CYP2C19, CYP3A4, UGT1A1, UGT1A3, and UGT1A9. The parent drug accounts for 89% of total detectable drug in the circulation, while AGI-16903 represents 10% of circulating total drug .
References
1) Yen et al. (2017), AG-221, A First-in-Class Therapy Targeting Acute Myeloid Leukemia Harboring Oncogenic IDH2 Mutations; Cancer Discov. 7 478 2) Amatangelo et al. (2017), Enasidenib induces acute myeloid leukemia cell differentiation to promote clinical response; Blood 130 732
Properties of Enasidenib
| Melting point: | 168-170°C |
| Boiling point: | 581.0±60.0 °C(Predicted) |
| Density | 1.477±0.06 g/cm3(Predicted) |
| storage temp. | -20°C |
| solubility | Soluble in DMSO (up to 25 mg/ml) |
| form | solid |
| pka | 14.70±0.29(Predicted) |
| color | White |
| Stability: | Stable for 1 year from date of purchase as supplied. Solutions in DMSO may be stored at -20°C for up to 3 months. |
Safety information for Enasidenib
| Signal word | Warning |
| Pictogram(s) |
 Exclamation Mark Irritant GHS07 |
| GHS Hazard Statements |
H302:Acute toxicity,oral H315:Skin corrosion/irritation H319:Serious eye damage/eye irritation H335:Specific target organ toxicity, single exposure;Respiratory tract irritation |
| Precautionary Statement Codes |
P280:Wear protective gloves/protective clothing/eye protection/face protection. P305+P351+P338:IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. Continuerinsing. |
Computed Descriptors for Enasidenib
New Products
(S)-3-Aminobutanenitrile hydrochloride 4-Methylphenylacetic acid N-Boc-D-alaninol N-BOC-D/L-ALANINOL Tert-butyl bis(2-chloroethyl)carbamate 3-Morpholino-1-(4-nitrophenyl)-5,6-dihydropyridin- 2(1H)-one Furan-2,5-Dicarboxylic Acid Tropic acid 1-Bromo-3,5-Di-Tert-Butylbenzene S-2-CHLORO PROPIONIC ACID ETHYL ISOCYANOACETATE 2-Bromo-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate 4-IODO BENZOIC ACID 3-NITRO-2-METHYL ANILINE 1-(2,4-DICHLOROPHENYL) ETHANAMINE (2-Hydroxyphenyl)acetonitrile 4-Bromopyrazole 2-(Cyanocyclohexyl)acetic acid 4-methoxy-3,5-dinitropyridine 1-(4-(aminomethyl)benzyl)urea hydrochloride 2-aminopropyl benzoate hydrochloride diethyl 2-(2-((tertbutoxycarbonyl)amino) ethyl)malonate tert-butyl 4- (ureidomethyl)benzylcarbamate Ethyl-2-chloro((4-methoxyphenyl)hydrazono)acetateRelated products of tetrahydrofuran
![2-(2-Chlorophenyl)-4-(3-(diMethylaMino)phenyl)-5-Methyl-1H-pyrazolo[4,3-c]pyridine-3,6(2H,5H)-dione](https://img.chemicalbook.in/CAS/20150408/GIF/1218942-37-0.gif)
You may like
-
 2033-24-1 98%View Details
2033-24-1 98%View Details
2033-24-1 -
 42831-50-5 5-METHYLISOXAZOLE-4-CARBOXYLIC ACID 98%View Details
42831-50-5 5-METHYLISOXAZOLE-4-CARBOXYLIC ACID 98%View Details
42831-50-5 -
 1975-50-4 98%View Details
1975-50-4 98%View Details
1975-50-4 -
 2-HYDROXY BENZYL ALCOHOL 98%View Details
2-HYDROXY BENZYL ALCOHOL 98%View Details
90-01-7 -
 2-Chloro-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate 221615-75-4 98%View Details
2-Chloro-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate 221615-75-4 98%View Details
221615-75-4 -
 61397-56-6 CIS BROMO BENZOATE 98%View Details
61397-56-6 CIS BROMO BENZOATE 98%View Details
61397-56-6 -
 14714-50-2 (2-Hydroxyphenyl)acetonitrile 98+View Details
14714-50-2 (2-Hydroxyphenyl)acetonitrile 98+View Details
14714-50-2 -
 118753-70-1 98+View Details
118753-70-1 98+View Details
118753-70-1