CHEMICAL AND PHYSICAL PROPERTIES
| Solubility | <1 mg/mL |
|---|
SAFETY INFORMATION
| Signal word | Warning |
|---|---|
| Pictogram(s) |
 Exclamation Mark Irritant GHS07 |
| GHS Hazard Statements |
H302:Acute toxicity,oral |
| Precautionary Statement Codes |
P280:Wear protective gloves/protective clothing/eye protection/face protection. P305+P351+P338:IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. Continuerinsing. |
COMPUTED DESCRIPTORS
| Molecular Weight | 583.0 g/mol |
|---|---|
| XLogP3 | 3.4 |
| Hydrogen Bond Donor Count | 1 |
| Hydrogen Bond Acceptor Count | 9 |
| Rotatable Bond Count | 7 |
| Exact Mass | 582.1394008 g/mol |
| Monoisotopic Mass | 582.1394008 g/mol |
| Topological Polar Surface Area | 119 Ų |
| Heavy Atom Count | 41 |
| Formal Charge | 0 |
| Complexity | 1050 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 2 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently-Bonded Unit Count | 1 |
| Compound Is Canonicalized | Yes |
PRODUCT INTRODUCTION
description
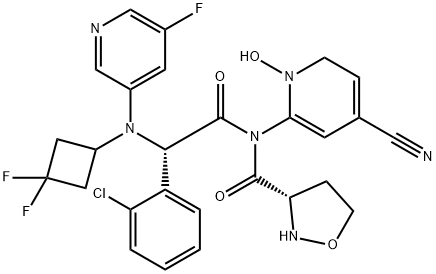
Ivosidenib is a tertiary carboxamide resulting from the formal condensation of the carboxy group of (2S)-1-(4-cyanopyridin-2-yl)-5-oxopyrrolidine-2-carboxylic acid with the secondary amino group of (2S)-2-(2-chlorophenyl)-N-(3,3-difluorocyclobutyl)-2-[(5-fluoropyridin-3-yl)amino]acetamide. It is approved by the FDA for the treatment of acute myeloid leukemia (AML) in patients with an isocitrate dehydrogenase-1 (IDH1) mutation. It has a role as an antineoplastic agent and an EC 1.1.1.42 (isocitrate dehydrogenase) inhibitor. It is a member of monochlorobenzenes, a cyanopyridine, a member of pyrrolidin-2-ones, an organofluorine compound, a tertiary carboxamide and a secondary carboxamide.