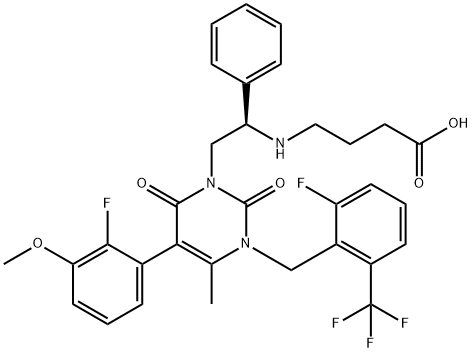
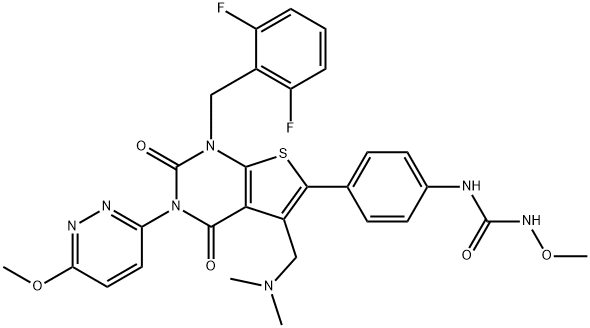
Relugolix
- CAS NO.:737789-87-6
- Empirical Formula: C29H27F2N7O5S
- Molecular Weight: 623.63
- MDL number: MFCD25976856
- EINECS: 237-099-7
- SAFETY DATA SHEET (SDS)
- Update Date: 2024-12-05 18:22:57
What is Relugolix?
Absorption
The Cmax and AUC of orally-administered relugolix increase proportionally following single doses - in contrast, with repeat dosing the AUC remains proportional to the dose while the Cmax increases greater than proportionally to the dose. Following the administration of 120mg once daily, the steady-state AUC and Cmax of relugolix were 407 (± 168) ng.hr/mL and 70 (± 65) ng/mL, respectively.
The absolute oral bioavailability of relugolix is approximately 12% and the median Tmax following oral administration is 2.25 hours.
Toxicity
Data regarding overdose of relugolix are unavailable.
Description
Relugolix (TAK-385) is a potent, orally active, nonpeptidic gonadotropin-releasing hormone (GnRH) antagonist. It possesses high affinity and potent antagonistic activity for human receptor (binding IC50=0.33 nM) and monkey receptor (IC50=0.32 nM) compared with TAK-013 (HY-100209). Relugolix is used for the study of sex-hormone-dependent diseases, such as including endometriosis, uterine fibroids and prostate cancer et al.
The Uses of Relugolix
Relugolix is a highly selective, oral, nonpeptide GnRH antagonist being investigated as a possible prostate cancer treatment
Background
Relugolix is a gonadotropin-releasing hormone (GnRH) receptor antagonist used in the treatment of several hormone-responsive conditions. It was first approved in Japan in 2019, under the brand name Relumina, for the symptomatic treatment of uterine fibroids, and more recently by the United States' FDA in 2020, under the brand name Orgovyx, for the treatment of advanced prostate cancer. This branded product was later approved by the European Commission on April 29, 2022. Relugolix has also been studied in the symptomatic treatment of endometriosis.
Relugolix is the first (and currently only) orally-administered GnRH receptor antagonist approved for the treatment of prostate cancer - similar therapies such as degarelix require subcutaneous administration - and therefore provides a less burdensome therapeutic option for patients who might otherwise require clinic visits for administration by healthcare professionals. In addition to its relative ease-of-use, relugolix was shown to be superior in the depression of testosterone levels when compared to leuprolide, another androgen deprivation therapy used in the treatment of prostate cancer. In May 2021, the FDA approved the combination product made up of relugolix, estradiol, and norethindrone under the market name Myfembree for the first once-daily treatment for the management of heavy menstrual bleeding associated with uterine fibroids in premenopausal women.
Indications
Relugolix is indicated for the treatment of adult patients with advanced prostate cancer. In a combination product with estradiol and norethindrone, relugolix is indicated for the once-daily treatment for the management of heavy menstrual bleeding associated with uterine fibroids in premenopausal women.
Mechanism of action
The mechanism of action of relugolix is as a Gonadotropin Releasing Hormone Receptor Antagonist, and Cytochrome P450 3A Inducer, and Cytochrome P450 2B6 Inducer, and Breast Cancer Resistance Protein Inhibitor, and P-Glycoprotein Inhibitor. The physiologic effect of relugolix is by means of Decreased GnRH Secretion.
Pharmacokinetics
Approximately 56% of patients achieved castrate-level testosterone concentrations (<50 ng/dL) by day 4 of therapy and 97% of patients maintain these levels through 48 weeks of therapy. Relugolix requires once-daily oral administration to maintain the desired testosterone concentrations.
Androgen deprivation therapies may prolong the QTc interval and should therefore be used with caution in patients having a high baseline risk of QTc prolongation, such as those with electrolyte abnormalities, congestive heart failure, or using other medications known to prolong the QTc interval. Based on its mechanism of action and data from animal studies, relugolix may result in fetal harm if administered to pregnant females - male patients with female partners should be advised to use effective contraception throughout therapy and for 2 weeks following cessation of therapy to prevent inadvertent fetal exposure.
Pharmacokinetics
Relugolix is a selective antagonist of the gonadotropin-releasing hormone receptor (GnRHR) (IC50 = 0.12 nM).A single oral administration of relugolix at a dose of 3 mg/kg has been found to suppress luteinizing hormone (LH) levels for more than 24 hours in castrated cynomolgus monkeys, indicating a long duration of action. The drug (80–160 mg/day) has been found to reduce testosterone levels to sustained castrate levels in men with once-daily administration.[8] Lower dosages (10–40 mg/day) are being studied in the treatment of endometriosis and uterine fibroids to achieve partial sex hormone suppression. The reasoning behind partial suppression for these conditions is to reduce the incidence and severity of menopausal symptoms such as hot flushes and to avoid bone mineral density changes caused by estrogen deficiency that can eventually lead to osteoporosis.
Clinical Use
Relugolix was first approved in Japan in 2019, under the brand name Relumina, for the symptomatic treatment of uterine fibroids, and more recently by the United States' FDA in 2020, under the brand name Orgovyx, for the treatment of advanced prostate cancer. Relugolix has also been studied in the symptomatic treatment of endometriosis.
Side Effects
Common side effects of Relugolix include: hot flashes; flushing of the skin; sweating; weight gain or loss of ability; pain in the muscles, back, joints or bones; fatigue; diarrhoea; constipation; difficulty sleeping; depression; and breast enlargement. More serious side effects include: dizziness; fainting; rapid heartbeat; or chest pain; swelling of the face, lips, mouth or tongue; difficulty breathing or swallowing; rash; measles; redness of the skin; chest pain or tightness in the chest; or pain in the arms, back, neck or jaw; sudden numbness or weakness of the face, arms, or legs (especially on one side of the body); sudden blurred consciousness; difficulty speaking or understanding; sudden difficulty seeing with one or both eyes; or sudden difficulty walking, walking, or seeing with one or both eyes. or sudden difficulty walking, dizziness, loss of balance or coordination, etc.
Synthesis
Relugolix is produced by the reaction of 6-(4-aminophenyl)-1-(2,6-difluorobenzyl)-5-((dimethylamino)methyl)-3-(6-methoxypyridazin-3-yl)thieno[2,3-d]pyrimidine-2,4(1H,3H)-dione and phenyl N-methoxycarbamate.
Metabolism
Relugolix is metabolized mainly by the CYP3A subfamily of P450 enzymes, with a smaller contribution by CYP2C8.
Properties of Relugolix
| Melting point: | 228 °C (decomp)(Solv: ethyl acetate (141-78-6); tetrahydrofuran (109-99-9)) |
| Density | 1.442±0.06 g/cm3(Predicted) |
| storage temp. | Store at -20°C |
| solubility | DMSO:20.0(Max Conc. mg/mL);32.1(Max Conc. mM) Ethanol:1.0(Max Conc. mg/mL);1.6(Max Conc. mM) |
| form | A crystalline solid |
| pka | 13.17±0.70(Predicted) |
| color | White to off-white |
Safety information for Relugolix
Computed Descriptors for Relugolix
| InChIKey | AOMXMOCNKJTRQP-UHFFFAOYSA-N |
| SMILES | N(C1=CC=C(C2SC3=C(C=2CN(C)C)C(=O)N(C2=NN=C(OC)C=C2)C(=O)N3CC2=C(F)C=CC=C2F)C=C1)C(NOC)=O |
Relugolix manufacturer
Radison Labs Private Limited
Exemed Pharmaceuticals (Oneiro Chemicals Pvt Ltd)
New Products
(S)-3-Aminobutanenitrile hydrochloride 4-Methylphenylacetic acid N-Boc-D-alaninol N-BOC-D/L-ALANINOL Tert-butyl bis(2-chloroethyl)carbamate 3-Morpholino-1-(4-nitrophenyl)-5,6-dihydropyridin- 2(1H)-one Furan-2,5-Dicarboxylic Acid Tropic acid 1-Bromo-3,5-Di-Tert-Butylbenzene S-2-CHLORO PROPIONIC ACID ETHYL ISOCYANOACETATE 2-Bromo-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate 4-IODO BENZOIC ACID 3-NITRO-2-METHYL ANILINE 1-(2,4-DICHLOROPHENYL) ETHANAMINE (2-Hydroxyphenyl)acetonitrile 4-Bromopyrazole 2-(Cyanocyclohexyl)acetic acid 4-methoxy-3,5-dinitropyridine 1-(4-(aminomethyl)benzyl)urea hydrochloride 2-aminopropyl benzoate hydrochloride diethyl 2-(2-((tertbutoxycarbonyl)amino) ethyl)malonate tert-butyl 4- (ureidomethyl)benzylcarbamate Ethyl-2-chloro((4-methoxyphenyl)hydrazono)acetateRelated products of tetrahydrofuran
You may like
-
 737789-87-6 Relugolix 99%View Details
737789-87-6 Relugolix 99%View Details
737789-87-6 -
 737789-87-6 98%View Details
737789-87-6 98%View Details
737789-87-6 -
 Relugolix 97%View Details
Relugolix 97%View Details -
 Relugolix 98%View Details
Relugolix 98%View Details -
 737789-87-6 Relugolix 99%View Details
737789-87-6 Relugolix 99%View Details
737789-87-6 -
 737789-87-6 98%View Details
737789-87-6 98%View Details
737789-87-6 -
 Relugolix 98%View Details
Relugolix 98%View Details
737789-87-6 -
 118753-70-1 98+View Details
118753-70-1 98+View Details
118753-70-1