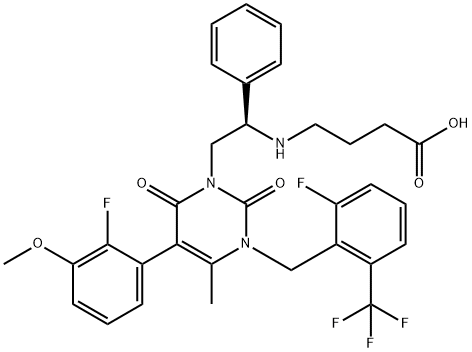
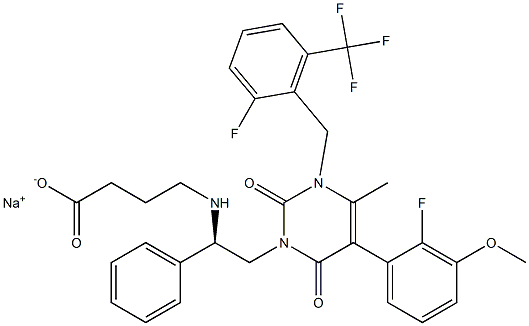
elagolix
- CAS NO.:834153-87-6
- Empirical Formula: C32H30F5N3O5
- Molecular Weight: 631.59
- MDL number: MFCD11973645
- SAFETY DATA SHEET (SDS)
- Update Date: 2024-11-19 23:02:33
What is elagolix?
Absorption
The Tmax of elagolix is reported as being 1.0 hours . The effect of a high-fat meal (relative to fasting) can result in a reduction of the AUC and Cmax by as much as 24% and 36%, respectively .
Toxicity
In case of overdose, monitor the patient for any signs or symptoms of adverse reactions and initiate appropriate symptomatic treatment, as needed . Common adverse reactions of elagolix include hot flush, headache, nausea, insomnia, mood alterations, amenorrhea, depression, anxiety, arthralgia, bone loss, changes in menstrual bleeding patterns, suicidal ideation and behavior, exacerbation of existing mood disorders, and/or hepatic transaminase elevations .
The recommended duration of use for elagolix is up to 24 months for the 150 mg once daily dose and up to six months for the 200 mg twice daily dose, as it causes a dose-dependent decrease in bone mineral density (BMD) . BMD loss is greater with increasing duration of use and may not be completely reversible after stopping treatment . For women with moderate hepatic impairment, the recommended dosage is 150 mg once daily for up to six months .
Description
Elagolix is a gonadotropin-releasing hormone(GnRH) receptor antagonist indicated for the management of moderate to severe pain associated with endometriosis. Elagolix is an orally administered,nonpeptide small molecule gonadotropin-releasing hormone(GnRH) receptor antagonist that inhibits endogenousGnRH signaling by binding competitively to GnRH receptors in the pituitary gland.
Elagolix results in dose-dependent suppression of luteinizing hormone(LH) and follicle-stimulating hormone(FSH) leading to decreased blood leveis of the ovarian sex hormones, estradiol and progesterone.Elagolix causes a dose-dependent decrease in bone mineral density (BMD).BMD loss is greater with increasing duration of use and may not be completely reversible after stopping treatment.
As milk production is dependent on at least minimal levels of estrogen, this product may reduce milk production.
The Uses of elagolix
Elagolix has a potential role for treating uterine bleeding associated to uterine myomas. Elagolix is also used in the methods for treatment of estrogen-dependent disorders including gonadotropin-releasing hormone (GnRH) antagonists in combination with add-back therapy.
Indications
Elagolix is a gonadotropin-releasing hormone (GnRH) receptor antagonist indicated for the management of moderate to severe pain associated with endometriosis .
Background
Elagolix has been used in trials studying the basic science and treatment of Endometriosis, Folliculogenesis, Uterine Fibroids, Heavy Uterine Bleeding, and Heavy Menstrual Bleeding. As of 24 July 2018, however, the U.S. Food and Drug Administration (FDA) approved AbbVie's elagolix under the brand name Orilissa as the first and only oral gonadotropin-releasing hormone (GnRH) antagonist specifically developed for women with moderate to severe endometriosis pain .
It has been determined that endometriosis is one of the most common gynecologic disorders in the United States . In particular, estimates suggest that one in ten women of reproductive age is affected by endometriosis and experience debilitating pain symptoms . Moreover, women who are affected by this condition can suffer for up to six to ten years and visit multiple physicians before receiving a proper diagnosis . Subsequently, as Orilissa (elagolix) was approved by the FDA under priority review , this expedited new approval gives healthcare professionals another valuable option for treating the potentially unmet needs of women who are affected by endometriosis, depending on their specific type and severity of endometriosis pain.
Preparation
Elagolix was a gonadotropin-releasing hormone antagonist. It was approved in 2018 by FDA via priority review for clinical treatment of endometriosis. Based on retrosynthetic analysis, five reported synthesis routes of elagolix were summarized. They were compared and evaluated in terms of step numbers, total yields, costs, synthetic conditions and manufacturing safety. At last, the synthetic method of d6-elagolix was introduced. This review would be beneficial to the future process researches of elagolix and its deuterium analogues.
Recent Progress in the Synthesis of Elagolix
Discovery of sodium R-(+)-4-{2-[5-(2-fluoro-3-methoxyphenyl)-3-(2-fluoro-6-[trifluoromethyl]benzyl)-4-methyl-2,6-dioxo-3,6-dihydro-2H-pyrimidin-1-yl]-1-phenylethylamino}butyrate (elagolix), a potent and orally available nonpeptide antagonist of the human gonadotropin-releasing hormone receptor
Definition
ChEBI: Elagolix is an organooxygen compound and an organonitrogen compound. It is functionally related to a gamma-amino acid.
Pharmacokinetics
During a three menstrual cycle study in healthy women, an elagolix 150 mg once daily regimen and a 200 mg twice daily regimen resulted in an ovulation rate of about 50% and 32%, respectively . In Phase 3 trials in women with endometriosis, elagolix caused a dose-dependent reduction in median estradiol concentrations to approximately 42 pg/mL for the 150 mg once daily regimen and 12 pg/mL for the 200 mg twice daily regimen .
Furthermore, the effect of elagolix on the QTc interval was investigated in a randomized, placebo- and positive-controlled, open-label, single-dose, crossover thorough QTc study in 48 healthy adult premenopausal women . Elagolix concentrations in subjects administered a single dose of 1200 mg was seventeen times higher than the concentration in subjects given elagolix 200 mg twice daily. Nevertheless, there was no clinically relevant prolongation of the QTc interval .
Metabolism
Elagolix is predominantly metabolized by the CYP3A family of isoenzymes despite participating in minor metabolic pathways with the CYP2D6, CYP2C8, and uridine glucuronosyl transferases (UGTs) enzymes as well . The primary metabolite of elagolix, referred to as NBI-61962 (R-(+)-4-{2-[5-(2-fluoro-3-hydroxy-phenyl)-3-(2-fluoro-6-trifluoromethyl-benzyl)-4-methyl-2,6-dioxo-3,6-dihydro-2H-pyrimidin-1-yl]-1-phenyl-ethylamino}-butyrate) , is not believed to possess any significant biologic activity due to its low plasma exposure and an observed potency that is exceptionally less than the parent elagolix compound (Ki value of 3.5 compared to 0.9 nM) .
Properties of elagolix
| Boiling point: | 728.6±70.0 °C(Predicted) |
| Density | 1.350 |
| storage temp. | Store at -20°C |
| solubility | DMSO : ≥ 100 mg/mL (158.33 mM) |
| pka | 4.40±0.10(Predicted) |
| form | Solid |
| color | White to off-white |
Safety information for elagolix
| Signal word | Danger |
| Pictogram(s) |
 Health Hazard GHS08 |
| GHS Hazard Statements |
H360:Reproductive toxicity |
| Precautionary Statement Codes |
P201:Obtain special instructions before use. P202:Do not handle until all safety precautions have been read and understood. P280:Wear protective gloves/protective clothing/eye protection/face protection. P308+P313:IF exposed or concerned: Get medical advice/attention. P405:Store locked up. P501:Dispose of contents/container to..… |
Computed Descriptors for elagolix
| InChIKey | HEAUOKZIVMZVQL-VWLOTQADSA-N |
| SMILES | C(O)(=O)CCCN[C@H](C1=CC=CC=C1)CN1C(=O)N(CC2=C(C(F)(F)F)C=CC=C2F)C(C)=C(C2=CC=CC(OC)=C2F)C1=O |
elagolix manufacturer
Exemed Pharmaceuticals (Oneiro Chemicals Pvt Ltd)
New Products
(S)-3-Aminobutanenitrile hydrochloride 4-Methylphenylacetic acid N-Boc-D-alaninol N-BOC-D/L-ALANINOL Tert-butyl bis(2-chloroethyl)carbamate N-octanoyl benzotriazole 3-Morpholino-1-(4-nitrophenyl)-5,6-dihydropyridin- 2(1H)-one Furan-2,5-Dicarboxylic Acid S-2-CHLORO PROPIONIC ACID ETHYL ISOCYANOACETATE 2-Bromo-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate 4-IODO BENZOIC ACID 3-NITRO-2-METHYL ANILINE 1-(2,4-DICHLOROPHENYL) ETHANAMINE (2-Hydroxyphenyl)acetonitrile 4-Bromopyrazole 5,6-Dimethoxyindanone 2-(Cyanocyclohexyl)acetic acid 4-methoxy-3,5-dinitropyridine 1-(4-(aminomethyl)benzyl)urea hydrochloride 2-aminopropyl benzoate hydrochloride diethyl 2-(2-((tertbutoxycarbonyl)amino) ethyl)malonate tert-butyl 4- (ureidomethyl)benzylcarbamate Ethyl-2-chloro((4-methoxyphenyl)hydrazono)acetateRelated products of tetrahydrofuran
![1-[2-fluoro-6-(trifluoromethyl)benzyl]-5-iodo-6-methylpyrimidine-2,4(1H,3H)-dione](https://img.chemicalbook.in/CAS/GIF/1150560-54-5.gif)
![Carbamic acid, N-[(1R)-2-[5-(2-fluoro-3-methoxyphenyl)-3-[[2-fluoro-6-(trifluoromethyl)phenyl]methyl]-3,6-dihydro-4-methyl-2,6-dioxo-1(2H)-pyrimidinyl]-1-phenylethyl]-, 1,1-dimethylethyl ester](https://img.chemicalbook.in/CAS/20180629/GIF/830346-51-5.gif)
You may like
-
![834153-87-6 Elagolix sodium
4-[[(1R)-2-[5-(2-Fluoro-3-methoxyphenyl)-3-[[2-fluoro-6- (trifluoromethyl)phenyl]methyl]-3,6-dihydro-4-methyl-2,6- dioxo-1(2H)-pyrimidinyl]-1-phenylethyl]amino]butanoic acid 96%](https://img.chemicalbook.in//ProductImageIndia/2024-03/Raw/a5457cd1-cdac-49c9-858e-7ca440d2b518.png) 834153-87-6 Elagolix sodium 4-[[(1R)-2-[5-(2-Fluoro-3-methoxyphenyl)-3-[[2-fluoro-6- (trifluoromethyl)phenyl]methyl]-3,6-dihydro-4-methyl-2,6- dioxo-1(2H)-pyrimidinyl]-1-phenylethyl]amino]butanoic acid 96%View Details
834153-87-6 Elagolix sodium 4-[[(1R)-2-[5-(2-Fluoro-3-methoxyphenyl)-3-[[2-fluoro-6- (trifluoromethyl)phenyl]methyl]-3,6-dihydro-4-methyl-2,6- dioxo-1(2H)-pyrimidinyl]-1-phenylethyl]amino]butanoic acid 96%View Details
834153-87-6 -
 834153-87-6 98%View Details
834153-87-6 98%View Details
834153-87-6 -
 1975-50-4 98%View Details
1975-50-4 98%View Details
1975-50-4 -
 2-HYDROXY BENZYL ALCOHOL 98%View Details
2-HYDROXY BENZYL ALCOHOL 98%View Details
90-01-7 -
 2-Chloro-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate 221615-75-4 98%View Details
2-Chloro-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate 221615-75-4 98%View Details
221615-75-4 -
 61397-56-6 CIS BROMO BENZOATE 98%View Details
61397-56-6 CIS BROMO BENZOATE 98%View Details
61397-56-6 -
 14714-50-2 (2-Hydroxyphenyl)acetonitrile 98+View Details
14714-50-2 (2-Hydroxyphenyl)acetonitrile 98+View Details
14714-50-2 -
 118753-70-1 98+View Details
118753-70-1 98+View Details
118753-70-1