Canagliflozin
- CAS NO.:842133-18-0
- Empirical Formula: C24H25FO5S
- Molecular Weight: 444.52
- MDL number: MFCD18251436
- EINECS: 695-192-1
- SAFETY DATA SHEET (SDS)
- Update Date: 2025-12-25 11:31:46
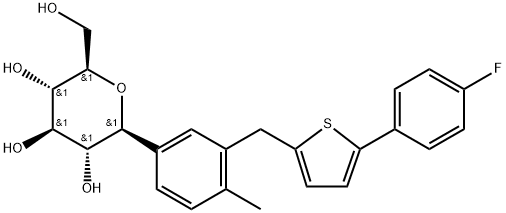
What is Canagliflozin?
Absorption
Bioavailability and steady-state
The absolute oral bioavailability of canagliflozin, on average, is approximately 65% . Steady-state concentrations are achieved after 4 to 5 days of daily dose administration between the range of 100mg to 300mg .
Effect of food on absorption
Co-administration of a high-fat meal with canagliflozin exerted no appreciable effect on the pharmacokinetic parameters of canagliflozin. This drug may be administered without regard to food. Despite this, because of the potential of canagliflozin to decrease postprandial plasma glucose excretion due to prolonged intestinal glucose absorption, it is advisable to take this drug before the first meal of the day .
Toxicity
Overdose information
If an overdose occurs, contact the Poison Control Center. Normal supportive measures should be taken, including the removal unabsorbed drug from the gastrointestinal tract, initiating clinical monitoring of the patient, and providing supportive treatment as deemed necessary. Canagliflozin has been removed in very small quantities after a 4-hour hemodialysis session. This drug is likely not dialyzable by peritoneal dialysis .
Pregnancy and lactation
Animal data has demonstrated that canagliflozin may cause adverse renal effects in a growing fetus. Data are insufficient at this time in determining a potential canagliflozin related risk for major birth defects or possible miscarriage in humans . There are known risks, however, of uncontrolled diabetes in pregnancy . Inform female patients taking canagliflozin of the potential risk, which is increased during the second and third trimesters. This drug is not recommended during nursing .
Mutagenesis and carcinogenicity
Canagliflozin was not found to be mutagenic in both metabolically activated and inactivated states in the Ames assay. Canagliflozin showed mutagenicity in laboratory mouse lymphoma assay, but only in the activated state. Canagliflozin was not found to be mutagenic in several in vivo assays performed on rats .
The carcinogenic risk of canagliflozin was assessed in 2-year studies completed in both CD1 mice and Sprague-Dawley rats. Canagliflozin was not shown to increase tumor incidence in mouse models given doses less than or equal to 14 times the exposure from a typical 300 mg dose in humans. Despite these negative findings in mice, the incidence of several tumors increased in mice, including Leydig cell tumors, renal tubular adenomas, and adrenal pheochromocytomas .
Description
In March 2013, the US FDA approved canagliflozin (JNJ-28431754; TA-7284) for treating type 2 diabetes in adults. Canagliflozin is the first US-approved sodium-glucose co-transporter (SGLT) inhibitor for treating type 2 diabetes. Inhibition of renal SGLT suppresses glucose reabsorption, which permits glucose excretion into urine and reduces hyperglycemia. Canagliflozin was discovered through structural modifications of phlorizin, a known inhibitor of renal glucose reabsorption. Early modifications of OH groups on the glucose moiety were insufficient to adequately impair hydrolysis by intestinal β-glucosidase. The introduction of the C-glucoside moiety, as in the clinical candidate dapagliflozin, afforded sufficient resistance to hydrolysis. Finally, incorporation of the thiophene moiety in canagliflozin provided improved potency for hSGLT2 (exclusive to kidney), IC50=2.2 nM, while offering significant selectivity over hSGLT1 (in kidney and heart), IC50=910 nM. Hyperglycemic, high-fat (HF) diet fed mice (KKstrain) that received a single 3 mg/kg oral dose of canagliflozin had a 48% reduction in blood glucose levels after 6 h versus vehicle-treated mice. Noteworthy in the multistep synthesis of canagliflozin is the stereoselective formation of the β-C-glucoside, which is accomplished by coupling the aryl lithium aglycone with 2,3,4,6-tetra-O-trimethylsilyl-β-Dgluconolactone followed by desilylation and stereoselective reduction with triethyl silane and boron trifluoride etherate.
Chemical properties
Pale Yellow Solid
Originator
Mitsubishi Tanabe Pharma (Japan)
The Uses of Canagliflozin
Canagliflozin is a selective SGLT-2 Inhibitor with IC50s of 2 nM, 3.7 nM, and 4.4 nM for mSGLT2, rSGLT2, and hSGLT2 in CHOK cells, respectively. It has been shown to dose-dependently reduce the calculated renal threshold for glucose excretion and increase urinary glucose excretion. Canagliflozin is a candidate for the treatment of type 2 diabetes and obesity.
Indications
This drug is used in conjunction with diet and exercise to increase glycemic control in adults diagnosed with type 2 diabetes mellitus .
Another indication for canagliflozin is the prevention of major cardiovascular events (myocardial infarction, stroke, or death due to a cardiovascular cause) in patients with type 2 diabetes, as well as hospitalization for heart failure in patients with type 2 diabetes.
In addition to the above, canagliflozin can be used to lower the risk of end-stage kidney disease and major increases in serum creatinine and cardiovascular death for patients with a combination of type 2 diabetes mellitus, diabetic nephropathy, and albuminuria.
It is important to note that this drug is not indicated for the treatment of type 1 diabetes mellitus or diabetic ketoacidosis .
Background
Canagliflozin, also known as Invokana, is a sodium-glucose cotransporter 2 (SGLT2) inhibitor used in the management of type 2 diabetes mellitus along with lifestyle changes including diet and exercise .
It was initially approved by the FDA in 2013 for the management of diabetes and later approved in 2018 for a second indication of reducing the risk of cardiovascular events in patients diagnosed with type 2 diabetes mellitus , .
Canagliflozin is the first oral antidiabetic drug approved for the prevention of cardiovascular events in patients with type 2 diabetes . Cardiovascular disease is the most common cause of death in these patients .
Definition
ChEBI: A C-glycosyl compound that is used (in its hemihydrate form) for treatment of type II diabetes via inhibition of sodium-glucose transport protein subtype 2.
brand name
Invokana
Clinical Use
Sodium-glucose co-transporter 2 inhibitor:
Treatment of type 2 diabetes
Drug interactions
Potentially hazardous interactions with other drugs
Antibacterials: concentration reduced by rifampicin.
Lipid-regulating drugs: avoid canagliflozin for 1 hour
before or 4-6 hours after bile acid sequestrants.
Mechanism of action
This drug increases urinary glucose excretion and decreases the renal threshold for glucose (RTG) in a dose-dependent manner. The renal threshold is defined as the lowest level of blood glucose associated with the appearance of detectable glucose in the urine. The result of canagliflozin administration is increased urinary excretion of glucose and less renal absorption of glucose, decreasing glucose concentration in the blood and improving glycemic control.
Metabolism
Canagliflozin is primarily metabolized by O-glucuronidation. It is mainly glucuronidated by UGT1A9 and UGT2B4 enzymes to two inactive O-glucuronide metabolites .
The oxidative metabolism of canagliflozin by hepatic cytochrome enzyme CYP3A4 is negligible (about 7%) in humans .
References
Nomura et al. (2010), Discovery of canagliflozin, a novel C-glucoside with thiophene ring, as sodium-dependent glucose cotransporter 2 inhibitor for the treatment of type 2 diabetes mellitus; J. Med. Chem., 53 6355 Shaw et al. (2011), Canagliflozin, a novel inhibitor of sodium glucose co-transporter 2, dose dependently reduces calculated renal threshold for glucose excretion and increases urinary glucose excretion in healthy subjects; Diabetes Obes. Metab., 13 669 Mantovani et al. (2020), Sodium-Glucose Cotransporter-2 Inhibitors for Treatment of Nonalcoholic Fatty Liver Disease: A Meta-Analysis of Randomized Controlled Trials; Metabolites, 11 22 Miller et al. (2020), Canagliflozin extends life span in genetically heterogeneous male but not female mice; JCI Insight, 5 e140019 Vilani et al. (2016), The diabetes medication Canagliflozin reduces cancer cell proliferation by inhibiting mitochondrial complex-I supported respiration; Mol. Metab., 5 1048 Xu et al. (2020), Inhibitory effects of canagliflozin on pancreatic cancer are mediated via the downregulation of glucose transporter-1 and lactate dehydrogenase A; Int. J. Oncol. 57 1223
Properties of Canagliflozin
| Melting point: | 68 - 72°C |
| Boiling point: | 642.9±55.0 °C(Predicted) |
| Density | 1.364 |
| storage temp. | -20°C |
| solubility | Soluble in DMSO (40 mg/ml) |
| form | solid |
| pka | 13.34±0.70(Predicted) |
| color | White |
| Stability: | Stable for 2 years from date of purchase as supplied. Solutions in DMSO may be stored at -20°C for up to 1 month. |
| CAS DataBase Reference | 842133-18-0 |
Safety information for Canagliflozin
Computed Descriptors for Canagliflozin
Canagliflozin manufacturer
New Products
4,4-Difluoropiperidine hydrochloride tert-butyl 9-methoxy-3-azaspiro[5.5]undecane-3-carboxylate Indole Methyl Resin N-Isopropylurea N,N-Dicyclohexylcarbodiimide(DCC) MELDRUMS ACID 5-METHYLISOXAZOLE-4-CARBOXYLIC ACID Magnessium Bis glycinate Zinc ascorbate 1-bromo-2-butyne 2-acetamidophenol 9(10H)-anthracenone Erythrosin B, 4-Piperidinopiperidine 2-((4-morpholinophenylamino) (methylthio) methylene) malononitrile 2,4-dihydroxybenzaldehyde 3-(4-morpholinophenylamino)-5-amino-1H-pyrazole-4-carbonitrile Methyl 2-methylquinoline-6-carboxylate 2,6-dichloro-4-nitropyridine 4-Bromo-2-chlorobenzonitrile 2-(benzylamino)acetic acid hydrochloride 4-(tert-Butoxycarbonylamino)but- 2-ynoic acid 3,4-dihydro-2H-benzo[b][1,4]dioxepine 1-Phenyl-1-cycloprppanecarboxylicacidRelated products of tetrahydrofuran

You may like
-
 Canagliflozin 98%View Details
Canagliflozin 98%View Details -
 842133-18-0 Canagliflozin 98%View Details
842133-18-0 Canagliflozin 98%View Details
842133-18-0 -
 Canagliflozin 98%View Details
Canagliflozin 98%View Details
842133-18-0 -
 Canagliflozin 99%View Details
Canagliflozin 99%View Details
842133-18-0 -
 Canagliflozin 98% (HPLC) CAS 842133-18-0View Details
Canagliflozin 98% (HPLC) CAS 842133-18-0View Details
842133-18-0 -
 Canagliflozin, Grade Standard: IPView Details
Canagliflozin, Grade Standard: IPView Details
842133-18-0 -
 Canagliflozin ChemicalView Details
Canagliflozin ChemicalView Details
842133-18-0 -
 Canagliflozin PowderView Details
Canagliflozin PowderView Details
842133-18-0
