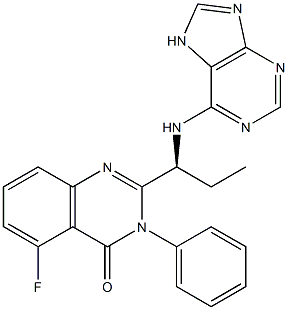
Radotinib
- CAS NO.:926037-48-1
- Empirical Formula: C27H21F3N8O
- Molecular Weight: 530.5
- MDL number: MFCD27956910
- SAFETY DATA SHEET (SDS)
- Update Date: 2024-11-19 15:53:33
What is Radotinib?
Description
Radotinib, an inhibitor of Bcr–Abl tyrosine kinase,was approved in January 2012 in Korea as a second-line treatment for chronic myeloid leukemia (CML). Radotinib is a TKI with a similar structure to the second-generation TKI, nilotinib, in which a pyridyl group has been replaced with a pyrazinemoiety. The in vitro activity of radotinib against a variety of tumor cell lines is disclosed in an issued patent. Radotinib was significantly more potent than imatinib in all of the cell lines tested. The synthesis of radotinib via amide coupling is described in the patent literature.
Originator
Il-Yang (Korea)
The Uses of Radotinib
Radotinib is tyrosine kinase inhibitor. In a biological study, it can induce cytotoxicity in c-KIT-positive malignancies including acute myeloid leukemia and small cell lung cancer in human making it potential target agent for treatment of such malignancies. It is a COVID19-related research product.
Indications
Radotinib (Supect(R), Il-Yang Pharmaceutical) is a Bcr–Abl inhibitor that was approved in South Korea in 2012 for the treatment of imatinib-resistant CML. Radotinib, which has a terminal 4-(pyridine-2-yl) pyrimidine moiety, was developed based on the previously approved Bcr–Abl inhibitors nilotinib. Radotinib has equivalent efficacy with that of other second-generation Bcr–Abl inhibitors and is well tolerated in chronic-phase CML patients. The lower cost of radotinib compared with other FDA-approved Bcr–Abl inhibitors makes it an attractive alternative for the treatment of CML in developing nations.
brand name
Supect
in vitro
radotinib couples to bcr-abl and reduce the phosphorylation of bcr-abl target protein crkl. the pre-clinical studies shows superiority of radotinib to imatinib in both wild-type and mutant bcr-abl1 positive cml cell lines. [1]
References
1. kim sh, menon h, jootar s et al. efficacy and safety of radotinib in chronic phase chronic myeloid leukemia patients with resistance or intolerance to bcr-abl1 tyrosine kinase inhibitors. haematologica. 2014 jul;99(7):1191-6.
Properties of Radotinib
| Density | 1.40±0.1 g/cm3(Predicted) |
| storage temp. | Store at -20°C |
| solubility | ≥26.55 mg/mL in DMSO; insoluble in EtOH; insoluble in H2O |
| form | solid |
| pka | 12.94±0.70(Predicted) |
| color | Light yellow to yellow |
Safety information for Radotinib
Computed Descriptors for Radotinib
New Products
4-AMINO-TETRAHYDRO-PYRAN-4-CARBOXYLIC ACID HCL 4-(Dimethylamino)tetrahydro-2H-pyran-4-carbonitrile 4-Aminotetrahydropyran-4-carbonitrile Hydrochloride (R)-3-Aminobutanenitrile Hydrochloride 3-((Dimethylamino)methyl)-5-methylhexan-2-one oxalate 1,4-Dioxa-8-azaspiro[4.5]decane 5-Bromo-2-nitropyridine Nimesulide BP Aceclofenac IP/BP/EP Diclofenac Sodium IP/BP/EP/USP Mefenamic Acid IP/BP/EP/USP Ornidazole IP Diclofenac Potassium THOMAIND PAPER PH 2.0 TO 4.5 1 BOX BUFFER CAPSULE PH 9.2 - 10 CAP SODIUM CHLORIDE 0.1N CVS ALLOXAN MONOHYDRATE 98% PLATINUM 0.5% ON 3 MM ALUMINA PELLETS (TYPE 73) LITHIUM AAS SOLUTION 2-Bromo-1-(bromomethyl)-3-chloro-5-nitrobenzene 2-Bromo-3-nitroaniline N-(3-Hydroxypropyl)-N-methylacetamide 3-Bromo-6-chloropyridazine 4-ethyl-3-nitrobenzoic acidRelated products of tetrahydrofuran
You may like
-
 Radotinib 95% CAS 926037-48-1View Details
Radotinib 95% CAS 926037-48-1View Details
926037-48-1 -
 1-Methyl-6-oxo-1,6-dihydropyridazine-3-carbonitrile 98%View Details
1-Methyl-6-oxo-1,6-dihydropyridazine-3-carbonitrile 98%View Details
99903-60-3 -
 1823368-42-8 98%View Details
1823368-42-8 98%View Details
1823368-42-8 -
 2-(3-(tert-butyl)phenoxy)-2-methylpropanoic acid 1307449-08-6 98%View Details
2-(3-(tert-butyl)phenoxy)-2-methylpropanoic acid 1307449-08-6 98%View Details
1307449-08-6 -
 Ethyl 3-(furan-2-yl)-3-hydroxypropanoate 25408-95-1 98%View Details
Ethyl 3-(furan-2-yl)-3-hydroxypropanoate 25408-95-1 98%View Details
25408-95-1 -
 2-Chloro-5-fluoro-1-methoxy-3-methylbenzene 98%View Details
2-Chloro-5-fluoro-1-methoxy-3-methylbenzene 98%View Details
1805639-70-6 -
 1784294-80-9 98%View Details
1784294-80-9 98%View Details
1784294-80-9 -
 Lithium ClavulanateView Details
Lithium ClavulanateView Details
61177-44-4