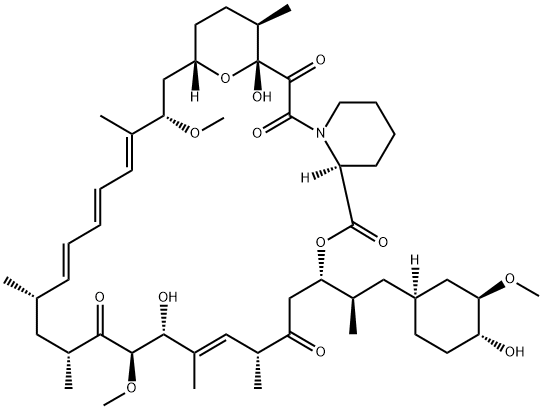
Temsirolimus
Synonym(s):42-[3-Hydroxy-2-(hydroxymethyl)-2-methylpropanoate]-rapamycin;CCI-779
- CAS NO.:162635-04-3
- Empirical Formula: C56H87NO16
- Molecular Weight: 1030.29
- MDL number: MFCD00934421
- EINECS: 686-177-0
- SAFETY DATA SHEET (SDS)
- Update Date: 2024-12-03 16:17:04

What is Temsirolimus?
Absorption
Infused intravenous over 30 - 60 minutes. Cmax is typically observed at the end of infusion
Toxicity
Temsirolimus has been administered to patients with cancer in phase 1 and 2 trials with repeated intravenous doses as high as 220 mg/m2. The risk of several serious adverse events, including thrombosis, bowel perforation, interstitial lung disease (ILD), seizure, and psychosis, is increased with doses of temsirolimus greater than 25 mg.
Description
While renal cell carcinoma (RCC) accounts for only 2 3% of all cancers, the 5-year survival rate for advanced RCC disease is only 5 10%, with approximately 13,000 deaths occurring annually (US statistics only). Immunotherapeutic cytokine options, such as IFN-αand IL-2, have traditionally been frontline treatments, but these agents are not efficacious in all patients and can cause serious side effects. In addition, bevacizumab, a monoclonal antibody against VEGF, has also demonstrated prolongation of PFS. The newest entry for this indication focuses on targets that are downstream from VEGF. Temsirolimus is an inhibitor of the serine/threonine kinase mTOR, which is the mammalian target of rapamycin. mTOR has been implicated in cell replication through control of the cell cycle translation of specific mRNAs. Inhibition of mTOR prevents phosphorylation of the 4E binding protein-1 and the 40S ribosomal protein S6 kinase that are responsible for cell cycle protein translation initiation; cell cycle arrest occurs as the result of termination of cell division from the G1 to the S phase. Disruption of mTOR signaling also has antiangiogenic effects that could be deemed essential in combating RCC, which is driven by unregulated angiogenesis. Temsirolimus is the 2,2-bis(hydroxymethyl)propionate ester of rapamycin (sirolimus), a macrolide fungicide isolated from the bacteria Streptomyces hygroscopicus. Similar to its parent sirolimus, temsirolimus interacts with mTOR through its complex with FK-506 binding protein 12.
Chemical properties
White Solid
Originator
Wyeth (US)
The Uses of Temsirolimus
Temsirolimus, a cell cycle inhibitor developed by Wyeth for the treatment of renal cell carcinoma, was launched in the US in 2007. Temsirolimus works by inhibiting mTOR (mammalian target of rapamycin)-driven cell proliferation. Temsirolimus is also being developed for the treatment of mantle cell lymphoma (PhIII) and also as mono- or combination therapy for the treatment of ovarian and endometrium cancer (PhII). Additionally, temsirolimus is being evaluated for the treatment of several other types of cancer as well as multiple sclerosis and rheumatoid arthritis.
The Uses of Temsirolimus
Temsirolimus (CCI-779) is a specific mTOR inhibitor with IC50 of 1.76 μM.
The Uses of Temsirolimus
Temsirolimus is a semisynthetic macrocyclic lactone prepared from rapamycin by selective alkylation of the 42-hydroxy group with a protected bis(dihydromethyl)propionic acid, followed by deprotection. Like all tacrolimus analogues, temsirolimus binds to receptor protein, FKBP12. The complex then binds to mTOR preventing it from interacting with target proteins. Temsirolimus is extensively cited in the literature with over 700 citations.
The Uses of Temsirolimus
Temsirolimus is a semisynthetic macrocyclic lactone prepared from rapamycin by selective acylation of the 42-hydroxy group with a protected bis(dihydromethyl)propionic acid, followed by deprotection. Like all tacrolimus analogues, temsirolimus binds to receptor protein, FKBP12. The complex then binds to mTOR preventing it from interacting with target proteins. Temsirolimus is extensively cited in the literature with over 700 citations.
Background
Temsirolimus is a derivative of sirolimus used in the treatment of renal cell carcinoma (RCC). It was developed by Wyeth Pharmaceuticals under the trade name Torisel. Temsirolimus was approved by the FDA in late May 2007 as well as the European Medicines Agency (EMEA) on November 2007.
Indications
For the treatment of renal cell carcinoma (RCC). Also investigated for use/treatment in breast cancer, lymphoma (unspecified), rheumatoid arthritis, and multiple myeloma.
brand name
Torisel
General Description
Temsirolimus is an esterified derivative of rapamycinand in a similar manner binds initially to the proteinFKBP-12(FK506-binding protein).This complexthen acts to inhibit the mammalian target of rapamycin(mTOR), a serine-threonine kinase that plays a crucial rolein cell division. It is somewhat unique in its method of kinaseinhibition, because it actually binds to an allostericmodulator of the kinase rather than just binding to theATP-binding site like most other kinase inhibitors.Temsirolimus is available as a 25-mg/mL injection forIV administration in the treatment of advanced RCC. Theagent is extensively metabolized and undergoes rapid hydrolysisof the ester function to give rapamycin that retainsactivity.Additional metabolism is mediated primarilyby CYP3A4 to give several hydroxylated and demethylatedmetabolites that are inactive. The agent and metabolites areeliminated primarily in the feces with half-lives of 17 and55 hours for temsirolimus and rapamycin, respectively.This agent, like rapamycin, possesses immunosuppressantproperties and there is an increased risk of infection.The most serious side effects are interstitial lung disease,perforation of the bowel, and acute renal failure althoughthese occur only rarely. The most commonly seen side effects are rash, weakness, mucositis, nausea, edema, andanorexia.
Biochem/physiol Actions
Temsirolimus is a specific inhibitor of mammalian target of rapamycin (mTOR) mTOR Complex 1 (mTORC1). Temsirolimus is an antiproliferative and antiangiogenic, the first-in-class mTOR inhibitor approved for the treatment of patients with advanced poor prognosis renal cell carcinoma.
Clinical Use
Protein kinase inhibitor:
Treatment of advanced renal cell carcinoma
Treatment of mantle cell lymphoma
Synthesis
While rufinamide may be prepared by several related routes, the preferred starting material is 2,6-difluorobenzyl azide. Reaction with either 2-propynoic acid or 2-chloroacrylonitrile affords 1-(2,6-difluorobenzyl)-1H-1,2,3-triazole-4-carboxylic acid or 1-(2,6-difluorobenzyl)- 1H-1,2,3-triazole-4-carbonitrile, respectively. For the carboxylic acid Shridhar Hegde and Michelle Schmidt intermediate, treatment with thionyl chloride followed by concentrated aqueous ammonium hydroxide or conversion to its methyl ester (methanol/sulfuric acid) with subsequent ammonolyis provides rufinamide.
Drug interactions
Potentially hazardous interactions with other drugs
Antibacterials: concentration increased by
clarithromycin and telithromycin - avoid;
concentration of both drugs increased with
erythromycin; concentration of active metabolite
reduced by rifampicin and rifabutin - avoid.
Antifungals: concentration increased of active
metabolite increased by ketoconazole - avoid;
concentration increased by fluconazole, itraconazole,
miconazole, micafungin, posaconazole and
voriconazole - avoid with itraconazole.
Antipsychotics: increased risk of agranulocytosis
with clozapine - avoid concomitant use.
Antivirals: concentration possibly increased by
atazanavir, boceprevir and lopinavir; concentration of
both drugs increased with telaprevir.
Calcium-channel blockers: concentration increased
by diltiazem; concentration of both drugs increased
with verapamil.
Ciclosporin: increased absorption of temsirolimus
- give temsirolimus 4 hours after ciclosporin;
temsirolimus concentration increased; long term
concomitant administration may be associated with
deterioration in renal function.
Cytotoxics: use crizotinib with caution.
Grapefruit juice: concentration of temsirolimus
increased - avoid.
Mycophenolate: concomitant use of mycophenolate
and sirolimus increases plasma levels of both
temsirolimus and mycophenolic acid.
Metabolism
Primarily metabolized by cytochrome P450 3A4 in the human liver. Sirolimus, an equally potent metabolite, is the primary metabolite in humans following IV infusion. Other metabolic pathways observed in in vitro temsirolimus metabolism studies include hydroxylation, reduction and demethylation.
Metabolism
Mainly metabolised by cytochrome P450 isoenzyme
CYP3A4 to 5 metabolites; sirolimus is the main active
metabolite, there is increased exposure to sirolimus
compared with temsirolimus due to longer half-life of
sirolimus.
Elimination is mainly in faeces; about 5% is recovered in
the urine.
storage
Store at -20°C
References
[1] yu k, toral-barza l, discafani c, et al. mtor, a novel target in breast cancer: the effect of cci-779, an mtor inhibitor, in preclinical models of breast cancer. endocrine-related cancer, 2001, 8(3): 249-258.
[2] frost p, moatamed f, hoang b, et al. in vivo antitumor effects of the mtor inhibitor cci-779 against human multiple myeloma cells in a xenograft model. blood, 2004, 104(13): 4181-4187.
Properties of Temsirolimus
| Melting point: | 99-101°C |
| Boiling point: | 1048.4±75.0 °C(Predicted) |
| Density | 1.21 |
| Flash point: | 587.8℃ |
| storage temp. | room temp |
| solubility | Soluble in chloroform, methanol. |
| form | powder |
| pka | 10.40±0.70(Predicted) |
| color | white to off-white |
| Merck | 14,9142 |
Safety information for Temsirolimus
| Signal word | Danger |
| Pictogram(s) |
 Health Hazard GHS08 |
| GHS Hazard Statements |
H360:Reproductive toxicity H413:Hazardous to the aquatic environment, long-term hazard |
| Precautionary Statement Codes |
P201:Obtain special instructions before use. P202:Do not handle until all safety precautions have been read and understood. P273:Avoid release to the environment. P280:Wear protective gloves/protective clothing/eye protection/face protection. P308+P313:IF exposed or concerned: Get medical advice/attention. P405:Store locked up. |
Computed Descriptors for Temsirolimus
| InChIKey | CBPNZQVSJQDFBE-FUXHJELOSA-N |
Temsirolimus manufacturer
Ralington Pharma
New Products
(S)-3-Aminobutanenitrile hydrochloride 4-Methylphenylacetic acid N-Boc-D-alaninol N-BOC-D/L-ALANINOL Tert-butyl bis(2-chloroethyl)carbamate N-octanoyl benzotriazole 3-Morpholino-1-(4-nitrophenyl)-5,6-dihydropyridin- 2(1H)-one Furan-2,5-Dicarboxylic Acid S-2-CHLORO PROPIONIC ACID ETHYL ISOCYANOACETATE 2-Bromo-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate 4-IODO BENZOIC ACID 3-NITRO-2-METHYL ANILINE 1-(2,4-DICHLOROPHENYL) ETHANAMINE (2-Hydroxyphenyl)acetonitrile 4-Bromopyrazole 5,6-Dimethoxyindanone 2-(Cyanocyclohexyl)acetic acid 4-methoxy-3,5-dinitropyridine 1-(4-(aminomethyl)benzyl)urea hydrochloride 2-aminopropyl benzoate hydrochloride diethyl 2-(2-((tertbutoxycarbonyl)amino) ethyl)malonate tert-butyl 4- (ureidomethyl)benzylcarbamate Ethyl-2-chloro((4-methoxyphenyl)hydrazono)acetateRelated products of tetrahydrofuran


You may like
-
 162635-04-3 Temsirolimus 98%View Details
162635-04-3 Temsirolimus 98%View Details
162635-04-3 -
 Temsirolimus 98%View Details
Temsirolimus 98%View Details
162635-04-3 -
 Temsirolimus 162635-04-3 98%View Details
Temsirolimus 162635-04-3 98%View Details
162635-04-3 -
 Temsirolimus CAS 162635-04-3View Details
Temsirolimus CAS 162635-04-3View Details
162635-04-3 -
 1975-50-4 98%View Details
1975-50-4 98%View Details
1975-50-4 -
 2-HYDROXY BENZYL ALCOHOL 98%View Details
2-HYDROXY BENZYL ALCOHOL 98%View Details
90-01-7 -
 14714-50-2 (2-Hydroxyphenyl)acetonitrile 98+View Details
14714-50-2 (2-Hydroxyphenyl)acetonitrile 98+View Details
14714-50-2 -
 118753-70-1 98+View Details
118753-70-1 98+View Details
118753-70-1