Zoledronic acid
Synonym(s):[1-hydroxy-2-(1H-imidazol-1-yl)ethane-1,1-diyl]bis(phosphonic acid) monohydrate;P,P′-[1-Hydroxy-2-(1H-imidazol-1-yl)ethylidene]bisphosphonic acid monohydrate;Zoledronate monohydrate;Zoledronic acid monohydrate
- CAS NO.:118072-93-8
- Empirical Formula: C5H10N2O7P2
- Molecular Weight: 272.09
- MDL number: MFCD00867791
- EINECS: 1806241-263-5
- SAFETY DATA SHEET (SDS)
- Update Date: 2024-10-25 16:21:11
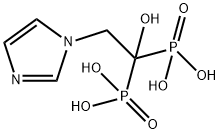
What is Zoledronic acid?
Absorption
A 4mg intravenous dose reaches a Cmax of 370±78.5ng/mL, with a Tmax of 0.317±0.014h, and an AUC of 788±181ng*h/mL. A 5mg intravenous dose reaches a Cmax of 471±76.1ng/mL, with a Tmax of 0.368±0.005h, and an AUC of 917±226ng*h/mL.
Toxicity
Patients experiencing an overdose may present with renal impairment, hypocalcemia, hypophosphatemia, and hypomagnesemia. Overdose should be managed through intravenous administration of the insufficient ions.
Description
Zoledronic acid is a white, crystalline powder that is available in vials for reconstitution for IV infusion over at least 15 minutes. It does not undergo metabolic transformation and does not inhibit CYP450 enzymes. Clearance of this agent is dependent on the patient's creatinine clearance, not on dose. Serum creatinine levels should be evaluated before every treatment. Zolendronic acid is contraindicated in patients with severe renal impairment.
Chemical properties
White Solid
Originator
Zometa,Novartis Pharma,Switz
The Uses of Zoledronic acid
Zoledronic acid induces apoptosis in osteoclasts by inhibiting enzymes of the mevalonate pathway and preventing the isoprenylation of small GTP-binding proteins such as Ras and Rho.
The Uses of Zoledronic acid
Bisphosphonate antiresorptive agent
The Uses of Zoledronic acid
bone resorption inhibitor
What are the applications of Application
Zoledronic acid, anhydrous is a protein kinase C activator with apoptotic effects
Background
Zoledronic acid, or CGP 42'446, is a third generation, nitrogen containing bisphosphonate similar to ibandronic acid, minodronic acid, and risedronic acid. Zoledronic acid is used to treat and prevent multiple forms of osteoporosis, hypercalcemia of malignancy, multiple myeloma, bone metastases from solid tumors, and Paget’s disease of bone. Zoledronic acid was first described in the literature in 1994.
Zoledronic acid was granted FDA approval on 20 August 2001.
Indications
Zoledronic acid is indicated to treat hypercalcemia of malignancy, multiple myeloma, bone metastases from solid tumors, osteoporosis in men and postmenopausal women, glucocorticoid induced osteoporosis, and Paget's disease of bone in men and women. Zoledronic acid is also indicated for the prevention of osteoporosis in post menopausal women and glucocorticoid induced osteoporosis.
Definition
ChEBI: An imidazole compound having a 2,2-bis(phosphono)-2-hydroxyethane-1-yl substituent at the 1-position.
Manufacturing Process
With stirring and under reflux, 8.6 g (0.053 mole) of imidazol-4-yl acetic acid hydrochloride, 7.1 ml of 85% phosphoric acid and 25 ml of chlorobenzene are heated to 100°C. Then 13.9 ml of phosphorus trichloride are added dropwise at 100°C, whereupon evolution of gas occurs. Over the course of 30 min a dense mass precipitates from the reaction mixture. The batch is heated for 3 hours to 100°C and the supernatant chlorobenzene is removed by decantation. With stirring and under reflux, the residual viscous mass is heated to the boil for 3 hours with 40 ml of 9 N hydrochloric acid. The batch is filtered hot with the addition of carbon and the filtrate is diluted with acetone, whereupon the crude 2-(imidazol-4-yl)-1-hydroxy-ethane-1,1- diphosphonic acid precipitates. This product is recrystallised from water. Melting point: 238-240°C (dec.).
brand name
Zometa (Novartis).
Therapeutic Function
Bone calcium regulator
Biological Functions
Zoledronic acid, a bisphosphonate, was approved by the U.S. FDA in 2001 for the treatment of hypercalcemia of malignancy, a metabolic complication that can be life-threatening. Hypercalcemia of malignancy can occur in up to 50% of patients diagnosed with advanced breast cancer, multiple myeloma, and nonsmall cell lung cancer. This condition arises when chemical moieties produced by the tumor cause overstimulation of osteoclasts. When there is an increase in bone degradation, there is a concomitant release of calcium into the plasma. When serum concentrations of calcium rapidly elevate, the kidneys are unable to handle the overload, and hypercalcemia results. This can lead to dehydration, nausea, vomiting, fatigue, and confusion. Zoledronic acid effectively decreases plasma calcium concentrations via inhibition of bone resorption (inhibition of osteoclastic activity and induction of osteoclast apoptosis). It also prevents the increase in osteoclastic activity caused by tumor-based stimulatory factors. Additionally zoledronic acid has been approved by the U.S. FDA for the treatment of multiple myeloma and bone metastases associated with solid tumor–based cancers (e.g., prostrate and lung). This agent is currently in late-stage clinical trials for the treatment and prevention of osteoporosis and, if approved, will be formulated as a 5-mg, once-yearly IV infusion.
Pharmacokinetics
Zoledronic acid is a third generation, nitrogen containing bisphosphonate that inhibits osteoclast function and prevents bone resorption. The therapeutic window is wide as patients are unlikely to suffer severe effects from overdoses and the duration of action is long. Patients should be counselled regarding the risk of electrolyte deficiencies, renal impairment, osteonecrosis of the jaw, atypical femoral fractures, bronchoconstriction, hepatic impairment, hypocalcemia, and embryo-fetal toxicity.
Clinical Use
Zoledronic acid is most commonly given to patients whose cancer is no longer responding to hormones, but it also may be given to prevent the bone thinning and weakening that results from hormonal treatments.
Drug interactions
Potentially hazardous interactions with other drugs
Other nephrotoxic drugs: use with caution as can
enhance nephrotoxicity
Metabolism
Zoledronic acid is not metabolized in vivio.
Metabolism
Zoledronic acid is not metabolised and is excreted unchanged via the kidney. Over the first 24 hours, 39 ± 16
% of the administered dose is recovered in the urine, while the remainder is principally bound to bone tissue.
storage
Store at +4°C
References
1) Green?et al. (1994),?Preclinical pharmacology of CGP 42446, a new, potent, heterocyclic bisphosphonate compound; J. Bone Miner. Res.,?9?745 2) Deeks and Perry (2008)?Zoledronic acid: a review of its use in the treatment of osteoporosis; Drugs Aging,?25?963 3) Perry and Figgitt (2004),?Zoledronic acid: a review of its use in patients with advanced cancer; Drugs,?64?1197 4) Koto?et al. (2010)?Zoledronic acid inhibits proliferation of human fibrosarcoma cells with induction of apoptosis and shows combined effects with other anticancer agents; Oncol. Rep.,?24?233 5) Tonyali?et al. (2010)?The role of zoledronic acid in the adjuvant treatment of breast cancer: current perspectives; Expert Opin. Pharmacother.,?11?2715
Properties of Zoledronic acid
| Melting point: | 193-2040C (dec) |
| Boiling point: | 764.0±70.0 °C(Predicted) |
| Density | 2.13±0.1 g/cm3(Predicted) |
| storage temp. | Sealed in dry,Room Temperature |
| solubility | Soluble in Water (up to 2 mg/ml). |
| form | solid |
| pka | 1.41±0.10(Predicted) |
| color | White or off-white |
| Stability: | Stable for 2 years from date of purchase as supplied. Solutions in distilled water may be stored at -20° for up to 2 months. |
| CAS DataBase Reference | 118072-93-8(CAS DataBase Reference) |
Safety information for Zoledronic acid
Computed Descriptors for Zoledronic acid
Zoledronic acid manufacturer
Shilpa Medicare Limited (SML)
Basil Drugs AND Pharmaceuticals Pvt Ltd
NATCO Pharma Limited
New Products
Tert-butyl bis(2-chloroethyl)carbamate 4-Methylphenylacetic acid N-Boc-D-alaninol N-BOC-D/L-ALANINOL N-octanoyl benzotriazole 3-Morpholino-1-(4-nitrophenyl)-5,6-dihydropyridin- 2(1H)-one Furan-2,5-Dicarboxylic Acid DIETHYL AMINOMALONATE HYDROCHLORIDE 1,1’-CARBONYLDIIMIDAZOLE R-2-BENZYLOXY PROPIONIC ACID 1,1’-CARBONYLDI (1,2-4 TRIAZOLE) N-METHYL INDAZOLE-3-CARBOXYLIC ACID (2-Hydroxyphenyl)acetonitrile 4-Bromopyrazole 5-BROMO-2CYANO PYRIDINE 5,6-Dimethoxyindanone 5-broMo-2-chloro-N-cyclopentylpyriMidin-4-aMine 2-(Cyanocyclohexyl)acetic acid 4-methoxy-3,5-dinitropyridine 1-(4-(aminomethyl)benzyl)urea hydrochloride 2-aminopropyl benzoate hydrochloride diethyl 2-(2-((tertbutoxycarbonyl)amino) ethyl)malonate tert-butyl 4- (ureidomethyl)benzylcarbamate Ethyl-2-chloro((4-methoxyphenyl)hydrazono)acetateRelated products of tetrahydrofuran
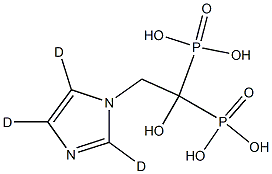

![[15N2,13C2]-Zoledronic Acid](https://img.chemicalbook.in/)
You may like
-
 118072-93-8 Zoledronic Acid 98%View Details
118072-93-8 Zoledronic Acid 98%View Details
118072-93-8 -
 Zoledronic Acid 98%View Details
Zoledronic Acid 98%View Details -
 118072-93-8 98%View Details
118072-93-8 98%View Details
118072-93-8 -
 Zoledronic Acid 118072-93-8 99%View Details
Zoledronic Acid 118072-93-8 99%View Details
118072-93-8 -
 Zoledronic Acid 98%View Details
Zoledronic Acid 98%View Details
118072-93-8 -
 Zoledronic Acid 118072-93-8 98%View Details
Zoledronic Acid 118072-93-8 98%View Details
118072-93-8 -
 Zoledronic Acid CAS 118072-93-8View Details
Zoledronic Acid CAS 118072-93-8View Details
118072-93-8 -
 Zoledronic acid 98% (HPLC) CAS 118072-93-8View Details
Zoledronic acid 98% (HPLC) CAS 118072-93-8View Details
118072-93-8