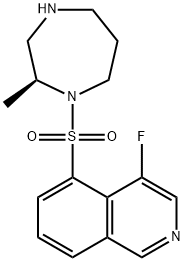
CAL-101
- CAS NO.:1146702-54-6
- Empirical Formula: C22H18FN7O
- Molecular Weight: 415.4230232
- SAFETY DATA SHEET (SDS)
- Update Date: 2024-11-19 20:33:22
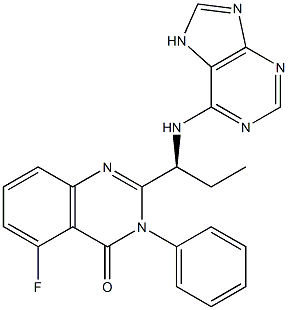
What is CAL-101?
Description
Idelalisib is an orally available selective and potent phosphatidylinositol 3-kinase δ (PI3 Kδ) inhibitor originally developed by Calistoga Pharmaceuticals, which was acquired by Gilead in April 2014. In July 2014, the drug was approved in the USA for the treatment of relapsed chronic lymphocytic leukemia as well as several oncology orphan drug designations. Since idelalisib specifically inhibits PI3Kd, which is expressed primarily in bloodcell lineages, the therapeutic effect is localized, limiting interference with PI3K isoform signaling that is critical to normal function of healthy cells.
Indications
Among the large groups of structural diverse lipid kinase inhibitors, especially against PI3Ks, idelalisib (Zydelig(R), Gilead Sciences) is the only inhibitor approved by FDA for the treatment of patients with relapsed chronic lymphocytic leukemia in combination with rituximab and patients with relapsed follicular B-cell non-Hodgkin lymphoma or small lymphocytic lymphoma.
Definition
ChEBI: Idelalisib is a member of the class of quinazolines that is 5-fluoro-3-phenylquinazolin-4-one in which the hydrogen at position 2 is replaced by a (1S)-1-(3H-purin-6-ylamino)propyl group. used for for the treatment of refractory indolent non-Hodgkin's lymphoma and relapsed chronic lymphocytic leukemia. It has a role as an antineoplastic agent, an apoptosis inducer and an EC 2.7.1.137 (phosphatidylinositol 3-kinase) inhibitor. It is a member of purines, an organofluorine compound, a member of quinazolines, an aromatic amine and a secondary amino compound.
brand name
Zydelig
General Description
Class: lipid kinase; Treatment: CLL, SLL, FL; Other name: CAL-101, GS-1101; Elimination half-life = 8.2 h; Protein binding > 84%
Pharmacokinetics
The recommended dose of idelalisib is 150 mg
orally twice a day, consistent with its elimination halflife is 8.2 h (Table 2). It is absorbed rapidly with a tmax of 1.5 h. Idelalisib is metabolized by aldehyde
oxidase and CYP3A to give a major metabolite GS-
563117 (Fig. 7), which is inactive against P110δ and
other isoforms.

Clinical Use
Phosphatidylinositol 3-kinase p110δ (PI3Kδ) inhibitor:
Treatment of chronic lymphocytic leukaemia (CLL)
and follicular lymphoma (FL)
Synthesis
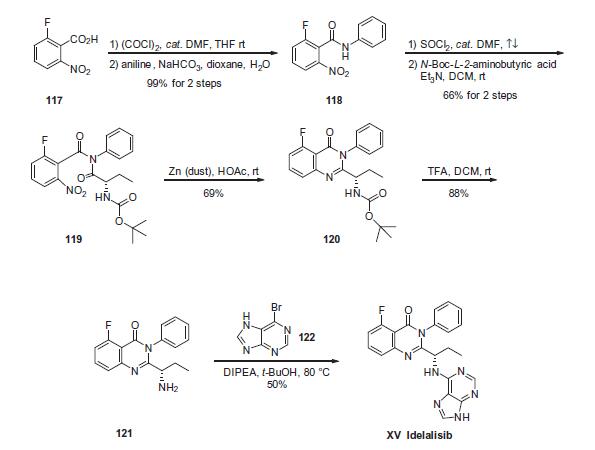
Commercial 2-fluoro-6-nitrobenzoic acid (117) was treated with oxalyl chloride in the presence of catalytic amount of N,Ndimethylformamide (DMF) in DCM to give the corresponding 2- fluoro-6-nitrobenzoyl chloride as a brown syrup, which was subsequently coupled with aniline under Schotten-Baumann conditions to yield 2-fluoro-6-nitro-N-phenylbenzamide 118 in 99% yield. Coupling of 118 with commercial N-Boc-2(S)-aminobutyric acid in the presence of Et3N in DCM generated imide 119 in 66% yield. Reductive cyclization of nitro imide 119 by means of zinc dust in acetic acid gave the cyclized quinazolinone 120 in 69% yield, which underwent immediate N-deprotection with TFA in DCM to furnish the corresponding free amine 121. Finally, a substitution reaction involving amine 121 and 6-bromopurine (122) in the presence of DIPEA in t-BuOH gave idelalisib (XV) as a solid in 50% yield.
Drug interactions
Potentially hazardous interactions with other drugs
Antibacterials: concentration reduced by rifampicin
- avoid.
Antidepressants: concentration possibly reduced by
St John’s wort - avoid.
Antiepileptics: concentration possibly reduced by
carbamazepine, fosphenytoin and phenytoin - avoid.
Antipsychotics: avoid with clozapine, increased
risk of agranulocytosis; avoid with pimozide and
quetiapine.
Metabolism
Idelalisib is metabolised mainly via aldehyde oxidase, and
to a lesser extent via CYP3A and UGT1A4. The primary
and only circulating metabolite, GS-563117, is inactive
against PI3Kδ.
Following a single 150 mg oral dose of [14C]-labelled
idelalisib, approximately 78% and 15% was excreted
in faeces and urine, respectively. Unchanged idelalisib
accounted for 23% of total radioactivity recovered in urine
over 48 hours and 12% of total radioactivity recovered in
faeces over 144 hours.
Safety information for CAL-101
New Products
Tert-butyl bis(2-chloroethyl)carbamate (S)-3-Aminobutanenitrile hydrochloride N-Boc-D-alaninol N-BOC-D/L-ALANINOL N-octanoyl benzotriazole 3,4-Dibenzyloxybenzaldehyde 4-Hydrazinobenzoic acid 1,1’-CARBONYLDIIMIDAZOLE R-2-BENZYLOXY PROPIONIC ACID 3-NITRO-2-METHYL ANILINE 4-IODO BENZOIC ACID 4-HYDROXY BENZYL ALCOHOL 4-(3-chloropropyl)morpholine phenylhydrazine hydrochloride (2-Hydroxyphenyl)acetonitrile 4-Bromopyrazole 5-BROMO-2CYANO PYRIDINE 5,6-Dimethoxyindanone 5-broMo-2-chloro-N-cyclopentylpyriMidin-4-aMine 4-methoxy-3,5-dinitropyridine 2-(Cyanocyclohexyl)acetic acid 2-aminopropyl benzoate hydrochloride 1-(4-(aminomethyl)benzyl)urea hydrochloride tert-butyl 4- (ureidomethyl)benzylcarbamateRelated products of tetrahydrofuran
You may like
-
 (9H-fluoren-9-yl)methyl (2,5-dioxopyrrolidin-1-yl) carbonate 82911-69-1 98.0%View Details
(9H-fluoren-9-yl)methyl (2,5-dioxopyrrolidin-1-yl) carbonate 82911-69-1 98.0%View Details
82911-69-1 -
 13057-17-5 95.0%View Details
13057-17-5 95.0%View Details
13057-17-5 -
![2-Nitro-8,9-dihydro-5H-benzo [7] annulen-7(6H)-one 98.0%](https://img.chemicalbook.in//Content/image/CP5.jpg) 2-Nitro-8,9-dihydro-5H-benzo [7] annulen-7(6H)-one 98.0%View Details
2-Nitro-8,9-dihydro-5H-benzo [7] annulen-7(6H)-one 98.0%View Details
740842-50-6 -
 4-bromoaniline 106-40-1 99.0%View Details
4-bromoaniline 106-40-1 99.0%View Details
106-40-1 -
 1421517-99-8 99.0%View Details
1421517-99-8 99.0%View Details
1421517-99-8 -
 5-bromo-2-chlorobenzoic acid 99.0%View Details
5-bromo-2-chlorobenzoic acid 99.0%View Details
21739-92-4 -
 2-methyl-5-nitrophenol 98.0%View Details
2-methyl-5-nitrophenol 98.0%View Details
5428-54-6 -
 15761-38-3 97.0%View Details
15761-38-3 97.0%View Details
15761-38-3