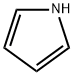
Pyrrolidine
Synonym(s):PRL;Pyrrolidine;Tetrahydropyrrole;Tetramethyleneimine
- CAS NO.:123-75-1
- Empirical Formula: C4H9N
- Molecular Weight: 71.12
- MDL number: MFCD00005249
- EINECS: 204-648-7
- SAFETY DATA SHEET (SDS)
- Update Date: 2025-09-30 18:11:34
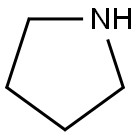
What is Pyrrolidine?
Chemical properties
Pyrrolidine appears as a colorless to pale yellow liquid with an ammonia-like odor. It is infinitely miscible with water and conventional organic solvents such as methanol, acetone, ether, and chloroform. It acts as a lachrymator.
Chemically, pyrrolidine behaves like a secondary amine in every respect. For example, it undergoes Leuckart –Wallach and Mannich reactions and is readily converted into an enamine. In the presence of a catalyst, such as platinum at 360°C or rhodium at 650°C, pyrrole is formed. In the presence of a copper catalyst, N-methylpyrrolidone is converted into N-methylpyrrolidine.
Physical properties
Pyrrolidine has a penetrating amine-type odor, reminiscent of ammonia and piperidine. It is easy to turn yellow when exposed to light or humid air, easily soluble in water and ethanol. It is nauseating and diffusive.
Occurrence
Reported found in beer, bread, wheat bread, salmon caviar, fish, milk, leaves and stalks of celery, Camembert cheese, Limburger cheese, Russian cheeses, tilsit cheese, other cheeses, caviar, raw fatty fish, beer, Finnish whiskey, white wine, red wine, coffee, radish, malt, roasted peanut, sweet corn and roasted barley.
The Uses of Pyrrolidine
Pyrrolidine is a flammable alkaline liquid that undergoes reactions typical of secondary amines. It is used to prepare pesticides and rubber accelerators and as a chemical intermediate (usually the hydrochloride form) in the pharmaceutical industry. There is relatively limited industrial exposure to this material.
Definition
ChEBI: Pyrrolidine is a cyclic amine whose five-membered ring contains four carbon atoms and one nitrogen atom; the parent compound of the pyrrolidine family. It is a saturated organic heteromonocyclic parent, a member of pyrrolidines and an azacycloalkane. It is a conjugate base of a pyrrolidinium ion.
Preparation
Pyrrolidine is formed by reduction of pyrrole. Via overall 5-endo-trig cyclizations of homoallylic tosylamides. Pyrrolidine can be produced from butanediol and ammonia, e.g., over an aluminum thorium oxide catalyst at 300°C or over a nickel catalyst at 200°C and 20 MPa under hydrogenation conditions. It can also be produced from THF and ammonia over aluminum oxide at 275-375°C.
What are the applications of Application
Pyrrolidine is a heterocyclic compound used as a building block in the synthesis of wide range of pharmaceutical compounds, namely matrix metalloprotein inhibitors (MMPIs) and aminopeptidase N inhibitors (APNIs). It has been used for the synthesis of N-benzoyl pyrrolidine from benzaldehyde via oxidative amination. It may be used as a catalyst for the synthesis of N-sulfinyl aldimines from carbonyl compounds and sulfonamides.
Pyrrolidine can also be used to synthesize:
Taddol-pyrrolidine phosphoramidite, a ligand for rhodium-catalyzed [2+2+2] cycloaddition of pentenyl isocyanate and 4- ethynylanisole.
H,4 PyrrolidineQuin-BAM (′PBAM′), a selective catalyst for the aza-Henry addition of nitroalkanes to aryl aldimines.
1,2,3,3a,4,9-Hexahydropyrrolo[2,1-b]quinazoline by reacting with o-aminobenzaldehyde.
Aroma threshold values
Detection: 20.2 ppm
Taste threshold values
Taste characteristics at 50 ppm: ammonia and fishy, amine-like with seaweed and shellfish nuances.
General Description
Pyrrolidine is a saturated heterocycliccompound having one nitrogenatom in a five-membered ring. It is a colorless to pale yellow liquid with an ammonia-like odor. It is found in certain plants andthe ring structure is present in manyalkaloids. Flash point 37°F. Density 0.85 g / cm3. Vapors heavier than air. Produces toxic oxides of nitrogen during combustion.
Air & Water Reactions
Highly flammable. Very soluble in water.
Reactivity Profile
Tetrahydro pyrrole neutralizes acids in exothermic reactions to form salts plus water. May be incompatible with isocyanates, halogenated organics, peroxides, phenols (acidic), epoxides, anhydrides, and acid halides. May generate hydrogen, a flammable gas, in combination with strong reducing agents such as hydrides. An explosion occurred when a mixture of Tetrahydro pyrrole, benzaldehyde, and propionic acid was heated in an attempt to form porphyrins.
Hazard
Flammable, dangerous fire risk. Toxic by ingestion and inhalation.
Health Hazard
The acute toxicity of pyrrolidine is moderateon test animals. It is somewhat less toxicthan pyrrole. The vapors are an irritant tothe eyes and respiratory tract. The liquid iscorrosive to the skin. Contact with the eyescan cause damage. The oral LD50 value inrats is 300 mg/kg, while the inhalation LC50value in mice is 1300 mg/m3/2 h (NIOSH1986).
Fire Hazard
Flammable/combustible material. May be ignited by heat, sparks or flames. Vapors may form explosive mixtures with air. Vapors may travel to source of ignition and flash back. Most vapors are heavier than air. They will spread along ground and collect in low or confined areas (sewers, basements, tanks). Vapor explosion hazard indoors, outdoors or in sewers. Runoff to sewer may create fire or explosion hazard. Containers may explode when heated. Many liquids are lighter than water.
Flammability and Explosibility
Highly flammable
Safety Profile
Poison by ingestion and intravenous routes. Moderately toxic by inhalation. Dangerous fire hazard when exposed to heat or flame; can react vigorously with oxidizing materials. To fight fire, use alcohol foam, CO2, dry chemical. When heated to decomposition it emits hghly toxic fumes of NOx.
Purification Methods
Dry pyrrolidine with BaO or sodium, then fractionally distil it, under N2, through a Todd column (p 11) packed with glass helices. [Beilstein 20 H 159, 20 I 36, 20 II 79, 20 III/IV 2072, 20/1 V 162.]
Properties of Pyrrolidine
| Melting point: | -63 °C |
| Boiling point: | 87-88 °C/760 mmHg (lit.) |
| Density | 0.852 g/mL at 25 °C (lit.) |
| vapor density | 2.45 (vs air) |
| vapor pressure | 128 mm Hg ( 39 °C) |
| refractive index | n |
| FEMA | 3523 | PYRROLIDINE |
| Flash point: | 37 °F |
| storage temp. | Store below +30°C. |
| solubility | water: miscible |
| form | Liquid |
| appearance | Colorless liquid |
| pka | 11.27(at 25℃) |
| color | Colorless to Almost colorless |
| PH | 12.9 (100g/l, H2O, 20℃) |
| Odor | at 0.10 % in propylene glycol. ammoniacal animal egg amine |
| explosive limit | 1.6-10.6%(V) |
| Water Solubility | Miscible with alcohol, ether, chloroform and water. |
| Sensitive | Air Sensitive |
| Merck | 14,8015 |
| JECFA Number | 1609 |
| BRN | 102395 |
| Dielectric constant | 8.3000000000000007 |
| Stability: | Stable; flammable. Incompatible with strong acids, strong oxidizing agents. |
| CAS DataBase Reference | 123-75-1(CAS DataBase Reference) |
| NIST Chemistry Reference | Pyrrolidine(123-75-1) |
| EPA Substance Registry System | Pyrrolidine (123-75-1) |
Safety information for Pyrrolidine
| Signal word | Danger |
| Pictogram(s) |
 Flame Flammables GHS02  Corrosion Corrosives GHS05  Exclamation Mark Irritant GHS07 |
| GHS Hazard Statements |
H225:Flammable liquids H314:Skin corrosion/irritation |
| Precautionary Statement Codes |
P210:Keep away from heat/sparks/open flames/hot surfaces. — No smoking. P280:Wear protective gloves/protective clothing/eye protection/face protection. P301+P312:IF SWALLOWED: call a POISON CENTER or doctor/physician IF you feel unwell. P303+P361+P353:IF ON SKIN (or hair): Remove/Take off Immediately all contaminated clothing. Rinse SKIN with water/shower. P305+P351+P338:IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. Continuerinsing. |
Computed Descriptors for Pyrrolidine
| InChIKey | RWRDLPDLKQPQOW-UHFFFAOYSA-N |
Pyrrolidine manufacturer
CEFA CILINAS BIOTICS PVT LTD
Antares Chem Private Limited
New Products
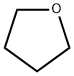
Indole Methyl Resin tert-butyl 9-methoxy-3-azaspiro[5.5]undecane-3-carboxylate Boc-His(Boc)-OH 2-CTC Resin 4-Chloro-7-tosy1-7Hpyrrolo[2,3-d]pyrimidine 5,7-Dibromo-1H-indole 2,5-dichloro-N-hydroxy-4,6-dimethylpyridine-3-carboximidamide 2,2-Dimethoxy-7-azaspiro[3.5]nonane hydrochloride 4-chloromethyl-5-methyl-1,3-dioxol-2-one (DMDO-Cl) R-2-BENZYLOXY PROPIONIC ACID 1,1’-CARBONYLDIIMIDAZOLE 1,1’-CARBONYLDI (1,2-4 TRIAZOLE) N-METHYL INDAZOLE-3-CARBOXYLIC ACID 4-((2-hydroxyethyl)thio)benzoic acid 1-(TERT-BUTOXYCARBONYL)-2-PYRROLIDINONE Methyl 6-methylnicotinate 3-Pyridineacrylic acid tert-Butyl carbazate TETRAHYDRO-2H-PYRAN-3-OL 2-((4-morpholinophenylamino) (methylthio) methylene) malononitrile 3-(4-morpholinophenylamino)-5-amino-1H-pyrazole-4-carbonitrile 2,4-dihydroxybenzaldehyde 1,3-Diethyl-1,3-Diphenylurea Methyl 2-methylquinoline-6-carboxylateRelated products of tetrahydrofuran

You may like
-
 Pyrrolidine, GR 99%+ CAS 123-75-1View Details
Pyrrolidine, GR 99%+ CAS 123-75-1View Details
123-75-1 -
 Pyrrolidine CASView Details
Pyrrolidine CASView Details -
 Pyrrolidine CAS 123-75-1View Details
Pyrrolidine CAS 123-75-1View Details
123-75-1 -
 Pyrrolidine pure CAS 123-75-1View Details
Pyrrolidine pure CAS 123-75-1View Details
123-75-1 -
 Pyrrolidine CAS 123-75-1View Details
Pyrrolidine CAS 123-75-1View Details
123-75-1 -
 Pyrrolidine extrapure AR CAS 123-75-1View Details
Pyrrolidine extrapure AR CAS 123-75-1View Details
123-75-1 -
 PYRROLIDINE For Synthesis CAS 123-75-1View Details
PYRROLIDINE For Synthesis CAS 123-75-1View Details
123-75-1 -
 Liquid Pyrrolidine, Grade Standard: Technical Grade, Packaging Type: DrumView Details
Liquid Pyrrolidine, Grade Standard: Technical Grade, Packaging Type: DrumView Details
123-75-1
