Prulifloxacin
- CAS NO.:123447-62-1
- Empirical Formula: C21H20FN3O6S
- Molecular Weight: 461.46
- MDL number: MFCD00864847
- EINECS: 819-932-1
- SAFETY DATA SHEET (SDS)
- Update Date: 2025-12-11 08:41:34
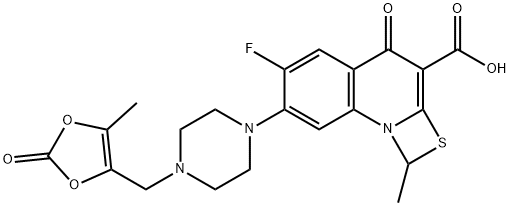
What is Prulifloxacin?
Description
Prulifloxacin was launched as the third fluoroquinone. It was introduced in Japan as an oral treatment for urinary tract infections (UTls), respiratory tract infections (RTls) and bacterial pneumoniae. It can be synthesized in 10 steps from commercially available 3,4-difluoroaniline. Key steps involve the cyclization of 6,7-difluoro-rl-hydroxy-2- thioquinoline-3carboxylic acid ethyl ester with 1 ,I-dibromomethane to give the corresponding thiazeto-[3,2a]quinoline. Aromatic nucleophilic substitution of the 7-fluoro atom with piperazine followed by hydrolysis of the ethyl ester and finally alkylation of the piperazinyl moiety with 4-(bromomethyl)-5-methyl-l ,bdioxol-Bone complete the synthesis. Prulifloxacin is a lipophilic prodrug, which is rapidly hydrolyzed to the corresponding Ndealkylated piperazine, NM 394, by paraoxonase type enzymes in blood and liver following intestinal absorption. The DNA gyrase inhibitor NM 394 accounts for all antimicrobial activity: it shows a similar or greater activity against gram-positive bacteria compared to ciprofloxacin, and a greater activity in the case of gram-negative bacteria. In clinical studies, prulifloxacin has shown good efficacy against UTls and RTls. The drug is mainly excreted in the urine and in the feces as unchanged NM 394, which has a plasma half-life of approximately 8 h. Phototoxicity in animal models is less severe than with other quinolones. Prulifloxacin is well tolerated with an adverse effect profile similar to that of other fluoroquinolones.
Chemical properties
Off-White Solid
Originator
Nippon Shinyaku (Japan)
The Uses of Prulifloxacin
Prulifloxacin is a synthetic chemotherapeutic antibiotic of the fluoroquinolone drug class. Prulifloxacin is a prodrug for active metabolite, Ulifloxacin. Antibacterial.
The Uses of Prulifloxacin
Fluoroquinoline antibacterial; prodrug for active metabolite, Ulifloxacin. Antibacterial.
The Uses of Prulifloxacin
Prulifloxacin Polymorph I is a crystallization form of a synthetic chemotherapeutic antibiotic of the fluoroquinolone drug class. Prulifloxacin is a prodrug for active metabolite, Ulifloxacin. Antibacterial.
What are the applications of Application
Prulifloxacin is a compound with broad spectrum antibacterial activity
Definition
ChEBI: Prulifloxacin is a quinolone antibiotic and a fluoroquinolone antibiotic.
brand name
Sword
Pharmaceutical Applications
A lipophilic prodrug which is very rapidly metabolized by esterase into ulifloxacin, a 6-fluoro, 7-piperazinyl thiazetoquinoline.
Ulifloxacin is moderately active against Staph. aureus (MIC 0.4–0.8 mg/L) and inactive against Str. pneumoniae (MIC 2–8 mg/L) as well as against Enterococcus spp. Against Enterobacteriaceae (MIC 0.05–0.8 mg/L) and Ps. aeruginosa (MIC 0.2–0.8 mg/L) activity is similar to that of ciprofloxacin. It is active against fastidious Gram-negative bacilli, but not against anaerobes and non-fermentative Gram-negative bacilli. Activity against Acinetobacter spp. is modest.
Prulifloxacin is rapidly converted into ulifloxacin and after 3 h is no longer detected in blood. In volunteers receiving a single oral dose, peak plasma concentrations of 0.68 mg/L (300 mg dose) to 1.88 mg/L (for 400 mg dose) were attained between 0.67 and 1.25 h. The mean apparent elimination half-life was 8 h and the mean cumulative elimination rate in urine within 48 h was 31–46%. Other inactive metabolites account for 7% of the dose. Half the administered dose is eliminated in feces within 72 h as ulifloxacin and 4% as prulifloxacin. Protein binding is 45%.
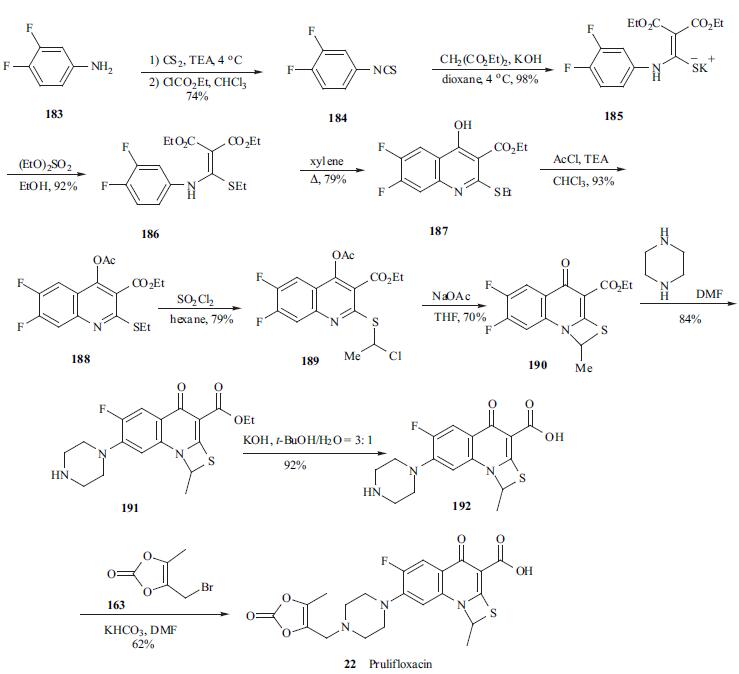
Synthesis
The synthesis of prulifloxacin (22)started with the treatment of 3,4- difluoroaniline (183) with carbon disulfide in the presence of TEA to give the triethylammonium dithiocarbamate, which by reaction with ethyl chloroformate and TEA in chloroform, was converted into isothiocyanate 184 in 74% yield. Reaction of 184 with diethyl malonate in the presence of KOH in dioxane yielded methylenemalonate 185 potassium salt, which was ethylated with ethyl sulfate in ethanol to give compound 186 in excellent yield. 6,7- Difluoroquinoline 187 was obtained with the highest yield and regioselectivity when precursor 186 was heated in refluxing xylene. To suppress the side reaction in the subsequent chlorination, quinoline 187 was acylated to give 188 with acetyl chloride in chloroform. Chlorination of 188 with sulfuryl chloride gave compound 189 in 79% yield. Compound 189 was treated with sodium acetate in THF to afford cyclized compound 190, which was condensed with piperazine in DMF to give compound 191. The hydrolysis of ester 191 with KOH in hot t-butanol gave free acid 192, which was finally condensed with 4-(bromomethyl)-5- methyl-1, 3-dioxol-2-one (163) by treatment of potassium bicarbonate in DMF to give prulifloxacin (22).
Properties of Prulifloxacin
| Melting point: | 211-214°C |
| Boiling point: | 633.2±65.0 °C(Predicted) |
| Density | 1.62±0.1 g/cm3(Predicted) |
| storage temp. | Sealed in dry,Store in freezer, under -20°C |
| solubility | 1 M NaOH: soluble25ML, clear, colorless (Solvent: 1 mg + 25 mL of NaOH) |
| form | White to yellow crystalline solid. |
| pka | 5.85±0.40(Predicted) |
| color | Off-White to Pale Yellow |
| λmax | 276nm(H2O)(lit.) |
| Merck | 14,7908 |
| CAS DataBase Reference | 123447-62-1(CAS DataBase Reference) |
Safety information for Prulifloxacin
| Signal word | Warning |
| Pictogram(s) |
 Exclamation Mark Irritant GHS07 |
| GHS Hazard Statements |
H302:Acute toxicity,oral H315:Skin corrosion/irritation H319:Serious eye damage/eye irritation H335:Specific target organ toxicity, single exposure;Respiratory tract irritation |
| Precautionary Statement Codes |
P261:Avoid breathing dust/fume/gas/mist/vapours/spray. P305+P351+P338:IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. Continuerinsing. |
Computed Descriptors for Prulifloxacin
Prulifloxacin manufacturer
New Products
Indole Methyl Resin tert-butyl 9-methoxy-3-azaspiro[5.5]undecane-3-carboxylate Boc-His(Boc)-OH 2-CTC Resin 4-Chloro-7-tosy1-7Hpyrrolo[2,3-d]pyrimidine 5,7-Dibromo-1H-indole 2,5-dichloro-N-hydroxy-4,6-dimethylpyridine-3-carboximidamide 2,2-Dimethoxy-7-azaspiro[3.5]nonane hydrochloride 4-chloromethyl-5-methyl-1,3-dioxol-2-one (DMDO-Cl) R-2-BENZYLOXY PROPIONIC ACID 1,1’-CARBONYLDIIMIDAZOLE 1,1’-CARBONYLDI (1,2-4 TRIAZOLE) N-METHYL INDAZOLE-3-CARBOXYLIC ACID 4-((2-hydroxyethyl)thio)benzoic acid 1-(TERT-BUTOXYCARBONYL)-2-PYRROLIDINONE Methyl 6-methylnicotinate 3-Pyridineacrylic acid tert-Butyl carbazate TETRAHYDRO-2H-PYRAN-3-OL 2-((4-morpholinophenylamino) (methylthio) methylene) malononitrile 3-(4-morpholinophenylamino)-5-amino-1H-pyrazole-4-carbonitrile 2,4-dihydroxybenzaldehyde 1,3-Diethyl-1,3-Diphenylurea Methyl 2-methylquinoline-6-carboxylateRelated products of tetrahydrofuran


You may like
-
 123447-62-1 Prulifloxacin 98%View Details
123447-62-1 Prulifloxacin 98%View Details
123447-62-1 -
 123447-62-1 98%View Details
123447-62-1 98%View Details
123447-62-1 -
 123447-62-1 98%View Details
123447-62-1 98%View Details
123447-62-1 -
 Prulifloxacin CAS 123447-62-1View Details
Prulifloxacin CAS 123447-62-1View Details
123447-62-1 -
 Prulifloxacin 98% (HPLC) CAS 123447-62-1View Details
Prulifloxacin 98% (HPLC) CAS 123447-62-1View Details
123447-62-1 -
 Prulifloxacin CAS 123447-62-1View Details
Prulifloxacin CAS 123447-62-1View Details
123447-62-1 -
 Pyridine 99.5% HPLC /UV SpectroscopyView Details
Pyridine 99.5% HPLC /UV SpectroscopyView Details
110-86-1 -
 Thiourea 99% ARView Details
Thiourea 99% ARView Details
62-56-6
