TEMPO
Synonym(s):2,2,6,6-Tetramethyl-1-piperidinyloxy, free radical;2,2,6,6-Tetramethylpiperidine 1-oxyl;2,2,6,6-Tetramethylpiperidine-1-oxyl (free radical);Tempo
- CAS NO.:2564-83-2
- Empirical Formula: C9H18NO*
- Molecular Weight: 156.25
- MDL number: MFCD00009599
- EINECS: 219-888-8
- SAFETY DATA SHEET (SDS)
- Update Date: 2025-12-07 19:09:50
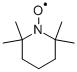
What is TEMPO?
Description
TEMPO, like DABCO, the Molecule of the Week for August 29, 2022, is one of those molecules best known by its acronym. TEMPO’s full name is 2,2,6,6-tetramethyl-1-piperidinyloxy.
TEMPO is also unusual in that it is a stable free radical. Because of its unpaired electron, its color is a bright red-orange, in contrast to similar molecular structures, which do not absorb light in the visible region.
TEMPO was first synthesized in 1959 by USSR chemists O. L. Lebedev and S. N. Kazarnovskii. They oxidized 2,2,6,6-tetramethylpiperidine1 with hydrogen peroxide in the presence of pertungstate ion (WO42–). The authors called the resulting product an “intermediate”; it is not clear whether they realized that it was a free radical. Three years later, another Soviet researcher, A. V. Il’yasov, published data on the effect of solvents on the electron paramagnetic resonance2 (EPR) spectra of some stable free radicals, including TEMPO, so its status had been established by then.
TEMPO has multiple uses in organic chemistry: as a catalyst in oxidation reactions and radical-mediated polymerizations and as an EPR marker or probe in chemical and biochemical systems. In a recent example, Abudureheman Wusiman and co-workers at Xingjiang Normal University (Urumqi, China) used it to promote the mono- and bisimidation of tertiary anilines. The purpose of the reaction was to create a straightforward route to symmetric and unsymmetric N-Mannich bases3 under mild conditions.
1. CAS Reg. No. 768-66-1.
2. Also known as electron spin resonance (ESR).
3. A Mannich base is a β-amino ketone; an N-Mannich base has a nitrogen atom in place of the intermediate carbon atom, e.g., R2NCH2NHC(O)R.
Description
2,2,6,6-Tetramethylpiperidine is a product that is used for the most diverse purposes because of its strong basicity, for example, as a light stabilizer for polyolefins, as a cocatalyst in olefin polymerizations (Ziegler catalysts), as a building block for the synthesis of pharmaceuticals and crop protection products, as a cocatalyst in the synthesis of dichloroacetyl chloride. The 4-unsubstituted 2.2.6,6-tetramethylpiperidine is generally prepared from the corresponding 4-oxo compound. It is known that triacetonamine can be converted by hydrazine to the hydrazone, which is then cleaved in the presence of alkali into 2,26,6-tetramethylpiperidine and nitrogen.
Chemical properties
orange crystals or powder
Characteristics
2,2,6,6-Tetramethylpiperidinooxy(TEMPO) has the characteristics of high yield, good selectivity, good stability, and recyclable use.
The Uses of TEMPO
TEMPO(2,2,6,6-Tetramethylpiperidinooxy) is a stable radical prepared through the oxidation of 2,2,6,6-tetramethylpiperidine. TEMPO has a wide range of applications including use as a free radical scavenger, a reagent in organic synthe sis and as a structural probe in electron spin resonance spectroscopy. TEMPO can also be used as a mediator in free radical polymerization.
The Uses of TEMPO
In organic chemistry as a radical trap, 2,2,6,6-Tetramethylpiperidinooxy can be used as a catalyst and in polymerization mediation.
The Uses of TEMPO
TEMPO can be used:
- As a catalytic oxidant for copper-catalyzed, greener oxidation of alcohols under aerobic conditions.
- As a catalytic oxidant in the iodobenzene diacetae oxidation of nerol to neral.
- For trapping the styrenyl radical generated from benzoyl peroxide during nitroxide-mediated radical polymerization of styrene.
- In the carboxylation of water-resistant nanofibrillated cellulose (NFC) films.
Copper(I)/TEMPO-catalyzed aerobic oxidation of primary alcohols to aldehydes with ambient air
What are the applications of Application
TEMPO is a catalyst employed in the oxidation of primary alcohols to aldehydes with NaOCl
General Description
For a synthetic protocol using NMP initiators, contributed by Prof. Karen Wooley, please visit our technology spotlight.
Purification Methods
Purify TEMPO by sublimation (33o, water aspirator) [Hay & Fincke J Am Chem Soc 109 8012 1987, Keana Chem Rev 78 37 1978].
Properties of TEMPO
| Melting point: | 36-38 °C(lit.) |
| Boiling point: | 193°C |
| Density | 1 g/cm3 |
| vapor pressure | 0.4 hPa (20 °C) |
| refractive index | 1.4350 (estimate) |
| Flash point: | 154 °F |
| storage temp. | Store below +30°C. |
| solubility | 9.7g/l |
| appearance | dark red to red-orange crystals or powder |
| appearance | Red-orange solids |
| form | Powder |
| color | Yellow to green |
| PH | 8.3 (9g/l, H2O, 20℃) |
| Water Solubility | Soluble in all organic solvents. Insoluble in water. |
| Merck | 14,9140 |
| BRN | 1422418 |
| Stability: | Stable. Incompatible with strong acids, strong oxidizing agents. Refrigerate. |
| CAS DataBase Reference | 2564-83-2(CAS DataBase Reference) |
| NIST Chemistry Reference | 1-Piperidinyloxy, 2,2,6,6-tetramethyl-(2564-83-2) |
| EPA Substance Registry System | 1-Piperidinyloxy, 2,2,6,6-tetramethyl- (2564-83-2) |
Safety information for TEMPO
| Signal word | Danger |
| Pictogram(s) |
 Corrosion Corrosives GHS05 |
| GHS Hazard Statements |
H314:Skin corrosion/irritation H412:Hazardous to the aquatic environment, long-term hazard |
| Precautionary Statement Codes |
P260:Do not breathe dust/fume/gas/mist/vapours/spray. P273:Avoid release to the environment. P280:Wear protective gloves/protective clothing/eye protection/face protection. P303+P361+P353:IF ON SKIN (or hair): Remove/Take off Immediately all contaminated clothing. Rinse SKIN with water/shower. P305+P351+P338:IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. Continuerinsing. |
Computed Descriptors for TEMPO
| InChIKey | RVWUHFFPEOKYLB-UHFFFAOYSA-N |
TEMPO manufacturer
JSK Chemicals
BTC Pharmaceuticals Technology India
Arown Pharma
GLR Innovations
Suvchem
ASM Organics
New Products
4,4-Difluoropiperidine hydrochloride tert-butyl 9-methoxy-3-azaspiro[5.5]undecane-3-carboxylate Indole Methyl Resin N-Isopropylurea N,N-Dicyclohexylcarbodiimide(DCC) MELDRUMS ACID 5-METHYLISOXAZOLE-4-CARBOXYLIC ACID Magnessium Bis glycinate Zinc ascorbate 1-bromo-2-butyne 2-acetamidophenol 9(10H)-anthracenone Erythrosin B, 4-Piperidinopiperidine 2-((4-morpholinophenylamino) (methylthio) methylene) malononitrile 2,4-dihydroxybenzaldehyde 3-(4-morpholinophenylamino)-5-amino-1H-pyrazole-4-carbonitrile Methyl 2-methylquinoline-6-carboxylate 2,6-dichloro-4-nitropyridine 4-Bromo-2-chlorobenzonitrile 2-(benzylamino)acetic acid hydrochloride 4-(tert-Butoxycarbonylamino)but- 2-ynoic acid 3,4-dihydro-2H-benzo[b][1,4]dioxepine 1-Phenyl-1-cycloprppanecarboxylicacidRelated products of tetrahydrofuran

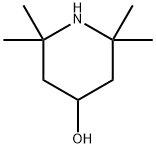
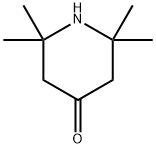

You may like
-
 2,2,6,6-Tetramethylpiperidinyloxy 98%View Details
2,2,6,6-Tetramethylpiperidinyloxy 98%View Details -
 2,2,6,6-Tetramethylpiperidine-1-Oxyl (TEMPO) pure CAS 2564-83-2View Details
2,2,6,6-Tetramethylpiperidine-1-Oxyl (TEMPO) pure CAS 2564-83-2View Details
2564-83-2 -
 Tempo 98% CAS 2564-83-2View Details
Tempo 98% CAS 2564-83-2View Details
2564-83-2 -
 2,2,6,6-Tetramethylpiperidine 1-Oxyl (Tempo) (Cas Number: 2564-83-2)View Details
2,2,6,6-Tetramethylpiperidine 1-Oxyl (Tempo) (Cas Number: 2564-83-2)View Details
2564-83-2 -
 Tempo Free Radical ,2,2,6,6-Tetramethylpiperidinyloxy ,CAS NO-2564-83-2View Details
Tempo Free Radical ,2,2,6,6-Tetramethylpiperidinyloxy ,CAS NO-2564-83-2View Details
2564-83-2 -
 2,2,6,6-Tetramethylpiperidine 1-Oxyl (Tempo, Free Radical)View Details
2,2,6,6-Tetramethylpiperidine 1-Oxyl (Tempo, Free Radical)View Details
2564-83-2 -
 TEMPO(2,2,6,6-Tetramethylpiperidine-1-oxyl)View Details
TEMPO(2,2,6,6-Tetramethylpiperidine-1-oxyl)View Details
2564-83-2 -
 2,2,6,6-Tetramethylpiperidinooxy, CAS NO:2564-83-2View Details
2,2,6,6-Tetramethylpiperidinooxy, CAS NO:2564-83-2View Details
2564-83-2
