Fluorine
Synonym(s):Coagulation factor II;F2;THRB
- CAS NO.:7782-41-4
- Empirical Formula: F2
- Molecular Weight: 38
- MDL number: MFCD00050975
- EINECS: 231-954-8
- SAFETY DATA SHEET (SDS)
- Update Date: 2025-12-10 11:56:18
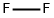
What is Fluorine?
Description
Fluorine is a highly toxic, pale yellow gas about 1.3 times as heavy as air at atmospheric temperature and pressure. When cooled below its boiling point (-306.8°F or -188.2°C), it is a liquid about 1.5 times as dense as water.
Chemical properties
Fluorine (F) is a chemical element(group VIla, halogens).It is a pale yellow,highly toxic, corrosive, flammable gas. It is a stable, extremely strong oxidant, which may react violently with combustible materials, including plastics, reducing agents, and organic material. It reacts with water to form corrosive acids. Fluorine is very toxic and may be fatal if inhaled. Fluorine reacts violently with many oxidising agents (e.g. perchlorates, peroxides, permanganates, chlorates, nitrates, chlorine, bromine, and fluorine), strong acids (hydrochloric, sulphuric, and nitric), organic compounds, combustible materials like oil and paper, hydrogen, bromine, iodine, and chemically active metals like, potassium, sodium, magnesium, and zinc.
Fluorine is the most electro negative of all elements and the most chemically energetic of all nonmetallic elements. Fluorine is a high-tonnage chemical that is used in production of fluorides, in synthesis of fluorocarbons,and as an oxidizer for rocket fuels. Because of its severe oxidizing characteristics, special permits are required for shipping of fluorine,and all containers,piping,and processing equipment used for fluorine service must be passivated prior to use. Thereafter, they must be designated for exclusive fluorine service.
Chemical properties
Fluorine is a yellow compressed, gas. Commonly shipped as a cryogenic gas. It has a characteris tic pungent odor; the odor threshold is 0.035 ppm.
Physical properties
Fluorine does not occur in a free state in nature, and because fluorine is one of the mostreactive elements, no chemical can free it from any of its many compounds. The reason forthis is that fluorine atoms are the smallest of the halogens, meaning the electron donated by ametal (or some nonmetals) are closer to fluorine’s nucleus and thus exert a great force betweenthe fluorine nuclei and the elements giving up one electron. The positive nuclei of fluorinehave a strong tendency to gain electrons to complete the outer shell, which makes it a strongoxidizer.
Because the fluorine atom has only nine electrons, which are close to the nucleus, thepositive nucleus has a strong tendency to gain electrons to complete its outer shell. As a gasits density (specific gravity) is 1.695, and as a liquid, its density is 1.108. Its freezing point is–219.61°C, and its boiling point is –188°C. Fluorine, as a diatomic gas molecule (F2), is paleyellow in color. Fluorine is the most electronegative nonmetallic element known (wants togain electrons) and is, therefore, the strongest oxidizing agent known.
Isotopes
There are a total of 16 isotopes of fluorine. Only one, F-19, is stable. It makesup 100% of the fluorine found on Earth. All the others are radioactive with half-livesranging from 2.5 milliseconds to 4.57100×10-22 years.
Origin of Name
From the Latin and French words for “flow,” fluere.
Occurrence
Fluorine is the 13th most abundant element on the Earth. It makes up about 0.06% of theEarth’s crust. Fluorine is widely distributed in many types of rocks and minerals, but neverfound in its pure form. Fluorine is as plentiful as nitrogen, chlorine, and copper, but lessplentiful than aluminum or iron.
The most abundant fluorine mineral is fluorite—calcium fluoride (CaF2)—which is oftenfound with other minerals, such as quartz, barite, calcite, sphalerite, and galena. It is mined in Cumberland, England, and in Illinois in the United States. Other minerals from which fluorineis recovered are fluorapatite, cryolite, and fluorspar, which are found in many countriesbut mainly in Mexico and Africa.
Today fluorine is produced by the electrolysis of potassium fluoride (KF), hydrofluoric acid(HF), and molten potassium acid fluoride (KHF2).
History
Fluorine was finally isolated in 1886 by Moisson. Fluorine occurs chiefly in fluorspar (CaF2) and cryolite (Na2AlF6), and is in topaz and other minerals. It is a member of the halogen family of elements, and is obtained by electrolyzing a solution of potassium hydrogen fluoride in anhydrous hydrogen fluoride in a vessel of metal or transparent fluorspar. Modern commercial production methods are essentially variations on the procedures first used by Moisson. Fluorine is the most electronegative and reactive of all elements. It is a pale yellow, corrosive gas, which reacts with practically all organic and inorganic substances. Finely divided metals, glass, ceramics, carbon, and even water burn in fluorine with a bright flame. Until World War II, there was no commercial production of elemental fluorine. The atom bomb project and nuclear energy applications, however, made it necessary to produce large quantities. Safe handling techniques have now been developed and it is possible at present to transport liquid fluorine by the ton. Fluorine and its compounds are used in producing uranium (from the hexafluoride) and more than 100 commercial fluorochemicals, including many well-known high-temperature plastics. Hydrofluoric acid is extensively used for etching the glass of light bulbs, etc. Fluorochlorohydrocarbons have been extensively used in airconditioning and refrigeration. However, in recent years the U.S. and other countries have been phasing out ozone-depleting substances, such as the fluorochlorohydrocarbons that have been used in these applications. It has been suggested that fluorine might be substituted for hydrogen wherever it occurs in organic compounds, which could lead to an astronomical number of new fluorine compounds. The presence of fluorine as a soluble fluoride in drinking water to the extent of 2 ppm may cause mottled enamel in teeth, when used by children acquiring permanent teeth; in smaller amounts, however, fluorides are said to be beneficial and used in water supplies to prevent dental cavities. Elemental fluorine has been studied as a rocket propellant as it has an exceptionally high specific impulse value. Compounds of fluorine with rare gases have now been confirmed. Fluorides of xenon, radon, and krypton are among those known. Elemental fluorine and the fluoride ion are highly toxic. The free element has a characteristic pungent odor, detectable in concentrations as low as 20 ppb, which is below the safe working level. The recommended maximum allowable concentration for a daily 8-hour time-weighted exposure is 1 ppm. Fluorine is known to have fourteen isotopes.
Characteristics
Fluorine reacts violently with hydrogen compounds, including water and ammonia. It alsoreacts with metals, such as aluminum, zinc, and magnesium, sometimes bursting into flames,and with all organic compounds, in some cases resulting in such complex fluoride compoundsas fluorocarbon molecules. It is an extremely active, gaseous element that combines spontaneouslyand explosively with hydrogen, producing hydrogen fluoride acid (HF), which is usedto etch glass. It reacts with most metals except helium, neon, and argon. It forms many differenttypes of “salts” when combining with a variety of metals. Fluorine, as a diatomic gas,is extremely poisonous and irritating to the skin and lungs, as are many fluoride compounds.Fluorine and its compounds are also corrosive.
The Uses of Fluorine
Fluorine is used in the manufacture of vari ous fluorocarbons and fluorides, as a rocketpropellant, and in many inorganic and or ganic syntheses.
The Uses of Fluorine
In manufacture of UF6 for nuclear power generation, of SF6 for dielectrics, of fluorinating and metal fluoride compounds.
The Uses of Fluorine
Probably the most common use of fluorine is its addition to municipal water supplies tohelp prevent tooth decay. Stannous (II) fluoride (SnF2) is added to the water in proportionsof about one part per million (1 ppm). In addition, many brands of toothpaste add stannousfluoride or other fluoride compounds to their product to help prevent tooth decay. Toothenamel degenerates overtime. Fluorine promotes remineralization, essentially making a formof new enamel called “fluorapatite,” which is resistant to decay.
Another popular use for the element fluorine is the plastic called Teflon. This is a fluoropolymerconsisting of long chainlike inert molecules of carbon linked chemically to fluorine.Teflon is useful as a coating for nonstick surfaces in cookware, ironing board covers, razorblades, and so forth.
Of great importance are the inert fluorocarbons, such as dichlorodifluoromethane (CF2Cl2)and chlorofluorocarbon compounds (CFCs) and their usage as gas propellants in spray cans(e.g., hair spray, deodorants, and paint). They are also used as coolants in air conditioning andrefrigeration (freon). The use of fluorinated carbon gases, known as fluorocarbons, in aerosolcans and refrigerants has been banned in the United States since 1978 because these gases diffuseinto the upper atmosphere and react to destroy the ozone gases found in the ozone layer.A reduced ozonosphere layer allows more ultraviolet radiation to filter to the Earth’s surface.Excessive strong ultraviolet radiation from the sun can be harmful to both plants and animals.The ozone layer filters out most of the harmful ultraviolet radiation.
When hydrogen and fluorine gases meet, they explode spontaneously and form hydrogenfluoride (HF), which, when dissolved in water, becomes hydrofluoric acid that is strongenough to dissolve glass. It is used to etch glass and to produce “frosted” light bulbs.
The artificial radioactive fluorine isotope F-18 emits positrons (positive electrons) that,when injected into the body, interact with regular negative electrons, and they annihilate each other, producing X-ray-like radiation. This medical procedure is performed in PositronEmission Topography (PET), in which the produced radiation generates a picture of the bodypart being examined. Since F-18 has a short half-life of about 110 minutes, there is littlechance of radiation damage to the patient.
Fluorine compounds are also used to reduce the viscosity of molten metals and slag byproductsso that they will flow more easily. In addition, fluorine is a component of therapeuticchemotherapy drugs used to treat a number of different types of cancer.
Definition
Nonmetallic halogen element in group 17 of the periodic table. An 9, aw 18.99840, valence of 1, no other stable isotopes, the most electronegative element and most powerful oxidizing agent known.
General Description
Fluorine is a pale yellow gas with a pungent odor. Fluorine is commonly shipped as a cryogenic liquid. Fluorine is toxic by inhalation and skin absorption. Contact with skin in lower than lethal concentrations causes chemical burns. Fluorine reacts with water to form hydrofluoric acid and oxygen. Fluorine is corrosive to most common materials. Fluorine reacts with most combustible materials to the point that ignition occurs. Under prolonged exposure to fire or intense heat the containers may violently rupture and rocket.
Air & Water Reactions
Water vapor will react combustibly with Fluorine; an explosive reaction occurs between liquid Fluorine and ice, after an intermediate induction period, [NASA SP-3037: 52(1967)]: If liquid air, which has stood for some time is treated with Fluorine, a precipitate is formed which is likely to explode. Explosive material is thought to be Fluorine Hydrate, [Mellor 2:11(1946-1947)].
Reactivity Profile
Propellant; ignites upon contact with alcohols, amines, ammonia, beryllium alkyls, boranes, dicyanogen, hydrazines, hydrocarbons, hydrogen, nitroalkanes, powdered metals, silanes, or thiols [Bretherick, 1979 p.174]; Aluminum powder and iodine in close contact will ignite spontaneously, Fluorine with metals requires added heat for ignition, [NFPA 491M]. Antimony is spontaneously flammable in Fluorine, chlorine, and bromine. With iodine, the reaction produces heat, which can cause flame or even an explosion if the quantities are great enough, [Mellor 9:379(1946-1947)]. The oxides of the alkalis and alkaline earths are vigorously attacked by Fluorine gas with incandescence, [Mellor 2:13(1946-1947)]. Fluorine causes aromatic hydrocarbons and unsaturated alkanes to ignite spontaneously, [Mellor 2, Supp. 1:55(1956)]. Fluorine vigorously reacts with arsenic and arsenic trioxide at ordinary temperatures, [Mellor 9:34(1946-1947)]. Bromine mixed with Fluorine at ordinary temperatures yields bromine trifluoride, with a luminous flame, [Mellor 2:12(1946-1947)]. Calcium silicide burns readily in Fluorine, [Mellor 6:663(1946-1947)]. The carbonates of sodium, lithium, calcium, and lead in contact with Fluorine are decomposed at ordinary temperatures with incandescence, [Mellor 2:13(1946-1947)]. A mixture of Fluorine and carbon disulfide ignites at ordinary temperatures, [Mellor 2:13(1946-1947)]. The reaction between Fluorine and carbon tetrachloride is violent and sometimes explosive, [Mellor 2, Supp. 1, 198(1956)]. The uncontrolled reaction between Fluorine and chlorine dioxide is explosive, [Mellor 2, Supp. 1, 532(1956)]. Fluorine and silver cyanide react with explosive violence at ordinary temperatures, [Mellor 2, Supp. 1:63(1956)]. Fluorine and sodium acetate produce an explosive reaction involving the formation of diacetyl peroxide, [Mellor 2, Supp. 1:56(1956)]. Selenium, silicon, or sulfur ignites in Fluorine gas at ordinary temperatures, [Mellor 2:11-13(1946-1947)]. Each bubble of sulfur dioxide gas led into a container of Fluorine produces an explosion, [Mellor 2:1(1946-1947)]. Fluorine and thallous chloride react violently, melting the product, [Mellor, Supp. 1:63(1956)].
Hazard
Powerful oxidizing agent; though nonflammable, it reacts violently with a wide range of both organic and inorganic compounds and thus is a dangerous fire and explosion risk in contact with such materials. Toxic by inhalation, extremely strong irritant to
Hazard
Many of the fluorine compounds, such as CFCs, are inert and nontoxic to humans. Butmany other types of compounds, particularly the salts and acids of fluorine, are very toxicwhen either inhaled or ingested. They are also strong irritants to the skin.
There is also danger of fire and explosion when fluorine combines with several elementsand organic compounds.
Poisonous fluoride salts are not toxic to the human body at the very low concentrationlevels used in drinking water and toothpaste to prevent dental decay.
Health Hazard
Poisonous; may be fatal if inhaled. Vapor extremely irritating. Contact may cause burns to skin and eyes. Chronic absorption may cause osteosclerosis and calcification of ligaments.
Health Hazard
reactions; highly irritating and corrosive to the eyes, skin, and mucous membranes. Toxicity The acute toxicity of fluorine is high. Even very low concentrations irritate the respiratory tract, and brief exposure to 50 ppm can be intolerable. High concentrations can cause severe damage to the respiratory system and can result in the delayed onset of pulmonary edema, which may be fatal. Fluorine is highly irritating to the eyes, and high concentrations cause severe injury and can lead to permanent damage and blindness. Fluorine is extremely corrosive to the skin, causing damage similar to second-degree thermal bums. Fluorine is not considered to have adequate warning properties. Chronic toxicity is unlikely to occur due to the corrosive effects of fluorine exposure. Fluorine has not been found to be carcinogenic or to show reproductive or developmental toxicity in humans.
Health Hazard
Fluorine is a severe irritant to the eyes, skin,and mucous membranes. In humans its irri tant effect on the eyes can be felt at a level of5 ppm in air. The acute toxicity of fluorine was found to be moderate in animals. Expo sure to this gas can cause respiratory distressand pulmonary edema. Chronic exposure canproduce mottled enamel of the teeth, calcifi-cation of ligaments, and injury to the lungs,liver, and kidney. The latter effects, however,were observed in animals at high concentra tions. The LC50 value in mice is 150 ppm foran exposure period of 1 hour. Human toxic ity data on fluorine are very limited.
Fire Hazard
May ignite other combustible materials (wood, paper, oil, etc.) Mixture with fuels may explode. Container may explode in heat of fire. Vapor explosion and poison hazard indoors, outdoors, or in sewers. Poisonous gas is produced in fire. Avoid contact with all oxidizable materials, including organic materials. Will react violently with water and most organic materials to produce heat and toxic fumes. Keep gas in tank, avoid exposure to all other materials.
Fire Hazard
Fluorine is not flammable, but is a very strong oxidizer, reacting vigorously with most oxidizable materials at room temperature, frequently with ignition. Water should not be used to fight fires involving fluorine
Flammability and Explosibility
Fluorine is not flammable, but is a very strong oxidizer, reacting vigorously with most oxidizable materials at room temperature, frequently with ignition. Water should not be used to fight fires involving fluorine.
Agricultural Uses
Fluorine is the lightest of the halogens, occurring naturally in fluorapatite, fluorite and cryolite. A pale yellow toxic gas, fluorine is made by electrolysis of potassium fluoride in liquid hydrogen fluoride. It is the most reactive, electronegative and oxidizing of all elements, and reacts with almost all elements, giving fluorides. It is used in rocket propulsion and in the production of uranium and fluorocarbons.
Materials Uses
Nickel, iron, aluminum, magnesium, copper, and certain of their alloys are quite satisfactory for handling fluorine at room temperature, for these are among the metals with which formation of a surface fluoride film retards further reaction.
Safety Profile
A poison gas. A skin, eye, and mucous membrane irritant. A most powerful caustic irritant to tissue. Mutation data reported. A very dangerous fire and explosion hazard. A powerful oxidizer. Reacts violently with many materials. with ammonia, cesium fluoride + fluorocarboxylic acids, cesium heptafluoropropoxide, 1or 2 fluoriminoperfluoropropane, graphite, halocarbons (e.g., carbon tetrachloride, chloroform, perfluorocyclobutane, iodo form, 1,2-d~hlorotetrafluoroethane), liquid hydrocarbons (e.g., anthracene, turpentine), hydrogen, hydrogen + oxygen, hydrogen fluoride + seleninyl fluoride + heat, nitric acid, silver cyanide, sulfur dioxide, carbon monoxide, sodium acetate, sodium bromate, stainless steel, water. Reacts to form explosive products with alkanes + oxygen (forms peroxides), cyano guanidine, perchloric acid (forms fluorine perchlorate gas), potassium chlorate (forms fluorine perchlorate gas), potassium hydroxide (forms potassium trioxide). Forms explosive mixtures with acetonitrile + chlorine fluoride, ice. Ignition or violent reaction on contact with acetylene, ceramic materials, covalent halides (e.g., chromyl chloride, phosphorus pentachloride, phosphorus trichloride, phosphorus trifluoride, boron trichloride, silicon tetrachloride), halogens (e.g., bromine, iodine, chlorine + spark or heating to 100°C), dcyanogen, gaseous hydrocarbons (e.g., town gas, methane, benzene), hydrogen halide gases or concentrated solutions (e.g., hydrogen bromide, hydrogen chloride, hydrogen iodide, hydrogen fluoride), metal acetylides and carbides (e.g., monocesium acetylide, cesium acetylide, lithium acetylide, rubidium acetylide, tungsten carbide, ditungsten carbide, zirconium dicarbide, uranium dicarbide), metal cyano complexes [e.g., potassium hexacyanoferrate(II), lead hexacyanoferrate(lII), potassium hexa cyanoferrate(III)], metal hydrides (e.g., copper hydride, potassium hydride, sodum hydride), metal iodides (e.g., lead iodide, calcium iodde, mercury iodide, potassium iodde), metals, metal salts, metal shcides (e.g., calcium disihcide, lithium hexasilicide), nickel(IV) oxide, nonmetals (e.g., boron, yellow or red phosphorus, selenium, tellurium, sdicon, carbon, charcoal, sulfur), oxygenated organic compounds (e.g., methanol, ethanol, 3-methyl butanol, acetaldehyde, trichloroacetaldehyde, acetone, lactic acid, benzoic acid, salicylic acid, ethyl acetate, methyl borate), nonmetal oxides (e.g., arsenic trioxide, nitrogen oxide, dinitrogen tetroxide), oxygen + polymers [e.g., phenol-formaldehyde resins (bakelite), polpacrylonitrile-butadiene (Buna N), polyamides (nylons), polychloropene (neoprene), polyethylene, polytrifluoroprop ylmethylsiloxane, poljrvin~7lchloride-vinyl acetate (Tygon), poljrvinylidene fluoride-hexafluoropropylene (Won), polyurethane foam, polymethyl methacrylate (Perspex), polytetrafluooethylene (Teflon)], sulfides (e.g., antimony trisulfide, carbon disulfide vapor, chromium (II) sulfide, hydrogen sulfide, barium sulfide, potassium sulfide, zinc sulfide, molybdenum sulfide), xenon + catalysts (e.g., nickel fluoride, silver difluoride, nickel(IⅡ) oxide, silver (I) oxide). Incandescent reaction with boron nitride, hexalithium dshcide + heat, metal borides, metal oxides (e.g., nickel(Ⅱ) oxide, alkali metal oxides, alkaline earth oxides), nitrogenous bases (e.g., aniline, dmethylamine, pyridne), gahc acid. Incompatible with cesium heptafluoro propoxide, cyanoguanid~ne, halocarbons,hexalithmm dishcide, seleninyl fluoride, hydrogen sulfide, oxygen, sodium acetate, sodium bromate, sodium dicyanamides, most organic matter, H-containing molecules, oxides of S, N, P, alkali metals,and alkaline earths. It reacts violently with halogen acids, hydrazine, ClO2, coke, cyanamide, cyanides, KNO3, (PbO + glycerol), CCl4, shcides, skates, trinitromethane, alkenes, alkyl benzenes, CS2, Cr(OCl)2, Al, T1, Sn, Sb, As, natural gas, liquid air, perfluoropropionyl fluoride, polyvinyl chloride acetate. Many reactions go on even at <-160°. Reacts with water or steam to produce heat and toxic and corrosive fumes. Used as one component of liquid rocket fuel and in chemical lasers. See also FLUORIDES.
Potential Exposure
Elemental fluorine is used in the con version of uranium tetrafluoride to uranium hexafluoride; in the synthesis of organic and inorganic fluorine com pounds; and as an oxidizer in rocket fuel.
Physiological effects
Fluorine gas is a powerful corrosive irritant and is highly toxic [I]. In one series of animal experiments, inhalation of acute exposures of 10 000 ppm for 5 minutes, 1000 ppm for 30 minutes, and 500 ppm for I hour produced 100 percent mortality in rats, mice, guinea pigs, and rabbits. Inhalation of 100 ppm for 7 hours produced wide variation in species mortality, ranging from 0 percent in guinea pigs to 96 percent in mice.
First aid
If this chemical gets into the eyes, remove anycontact lenses at once and irrigate immediately for at least15 min, occasionally lifting upper and lower lids. Seek medical attention immediately. If this chemical contacts theskin, remove contaminated clothing and wash immediatelywith soap and water. Seek medical attention immediately. Ifthis chemical has been inhaled, remove from exposure,begin rescue breathing (using universal precautions, including resuscitation mask) if breathing has stopped and CPR ifheart action has stopped. Transfer promptly to a medicalfacility. When this chemical has been swallowed, get medical attention. Give large quantities of water and inducevomiting. Do not make an unconscious person vomit. Iffrostbite has occurred, seek medical attention immediately;do NOT rub the affected areas or flush them with water. Inorder to prevent further tissue damage, do NOT attempt toremove frozen clothing from frostbitten areas. If frostbitehas NOT occurred, immediately and thoroughly wash contaminated skin with soap and water.
Environmental Fate
Fluorine remains persistent in the environment. In water, fluorides attach to aluminum in freshwater and calcium and magnesium in seawater and settle into the sediment. Fluorides may be taken up from soil and accumulate in plants or they may be deposited on the upper parts of the plants. The amount of fluoride taken up by plants depends on the type of plant, the nature of the soil, and the amount and form of fluoride in the soil. Levels of fluorides in surface water average about 0.2 ppm, while well water levels range from 0.02 to 1.5 ppm. The 15 000 water systems serving about 162 million people in the USA are fluoridated in the range of 0.7–1.2 ppm.
Storage
Work with fluorine requires special precautions and protective equipment and should be carried out only by specially trained personnel. Fluorine will react with many materials normally recommended for handling compressed gases.
Shipping
UN1045 Fluorine, compressed, Hazard Class: 2.3; Labels: 2.3-Poisonous gas, 5.1-Oxidizer, 8-Corrosive material, Inhalation Hazard Zone A. Cylinders must be transported in a secure upright position, in a well-ventilated truck. Protect cylinder and labels from physical damage. The owner of the compressed gas cylinder is the only entity allowed by federal law (49CFR) to transport and refill them. It is a violation of transportation regulations to refill compressed gas cylinders without the express written per mission of the owner.
Purification Methods
Pass the gas through a bed of NaF at 100o to remove HF and SiF4. [For description of stills used in fractional distillation, see Greenberg et al. J Phys Chem 65 1168 1961; Stein et al. Purification of Fluorine by Distillation, Argonne National Laboratory, ANL-6364 1961 (from Office of Technical Services, US Dept of Commerce, Washington 25).] HIGHLY TOXIC.
Toxicity evaluation
Fluorine-containing compounds are diverse and the specifics of
toxicity depend upon their reactivity, structure, and ability to
release fluoride ions. Ingested fluoride initially acts locally on
the intestinal mucosa, where it forms hydrofluoric acid. Once
released, fluoride ions combined with blood calcium forming
calcium fluoride producing hypocalcemia. Fluoride at high
doses can stimulate osteoblasts and inhibit osteoclasts. Inorganic
fluoride inhibits enzymes requiring metal ion cofactors
which inhibit ATP production in the mitochondrial electron
transport chain system of the cell.
Although the exact mechanism of dental fluorosis is
unknown, it is generally believed to be due to a fluorideinduced
delay in the hydrolysis and removal of the enamel
amelogenin matrix proteins during enamel maturation and
subsequent effects on crystal growth. In one proposed mechanism,
fluoride indirectly inhibits amelogeninase, a calciumdependent
metalloenzyme, by binding to calcium thereby
decreasing the calcium availability and the activation of
amelogeninases.
Incompatibilities
Fluorine is an extremely powerful oxi dizing gas. Keep away from heat, water, nitric acid, oxidi zers, organic compounds. Containers may explode if heated. Reacts violently with reducing agents; ammonia, all combustible materials, metals (except the metal containers in which it is shipped). Reacts violently with H2O to form hydrofluoric acid, oxygen and ozone. The most potent oxidizer.
Waste Disposal
Return refillable compressed gas cylinders to supplier. Fluorine may be combusted by means of a fluorine-hydrocarbon air burner followed by a caustic scrubber and stack. Consult with environmental regulatory agencies for guidance on acceptable disposal practices. Generators of waste containing this contaminant (≥100 kg/mo) must conform with EPA regulations gov erning storage, transportation, treatment, and waste disposal.
GRADES AVAILABLE
Fluorine is available at a minimum purity of 97 percent.
Properties of Fluorine
| Melting point: | -220°C |
| Boiling point: | -188°C |
| Density | 1.695(15℃) |
| vapor pressure | >760 mmHg at 20 °C |
| refractive index | 1.000195 |
| storage temp. | -20°C |
| solubility | reacts with H2O |
| form | pale yellow gas |
| color | pale |
| Odor | Strong ozone-like odor detectable at 0.1 to 0.2 ppm |
| Water Solubility | reacts |
| Dielectric constant | 1.5(-201℃) |
| Exposure limits | TLV-TWA 1 ppm (~2 mg/m3) (ACGIH and
MSHA), 0.1 ppm (OSHA); IDLH 25 ppm
(NIOSH). |
| Stability: | Stable. Extremely strong oxidant which may react violently with combustible materials, including plastics, reducing agents and organic material. Reacts with water to form corrosive acids. |
| CAS DataBase Reference | 7782-41-4(CAS DataBase Reference) |
| NIST Chemistry Reference | Fluorine(7782-41-4) |
| EPA Substance Registry System | Fluorine (7782-41-4) |
Safety information for Fluorine
Computed Descriptors for Fluorine
Fluorine manufacturer
New Products
4,4-Difluoropiperidine hydrochloride tert-butyl 9-methoxy-3-azaspiro[5.5]undecane-3-carboxylate Indole Methyl Resin N-Isopropylurea N,N-Dicyclohexylcarbodiimide(DCC) MELDRUMS ACID 5-METHYLISOXAZOLE-4-CARBOXYLIC ACID Magnessium Bis glycinate Zinc ascorbate 1-bromo-2-butyne 2-acetamidophenol 9(10H)-anthracenone Erythrosin B, 4-Piperidinopiperidine 2-((4-morpholinophenylamino) (methylthio) methylene) malononitrile 2,4-dihydroxybenzaldehyde 3-(4-morpholinophenylamino)-5-amino-1H-pyrazole-4-carbonitrile Methyl 2-methylquinoline-6-carboxylate 2,6-dichloro-4-nitropyridine 4-Bromo-2-chlorobenzonitrile 2-(benzylamino)acetic acid hydrochloride 4-(tert-Butoxycarbonylamino)but- 2-ynoic acid 3,4-dihydro-2H-benzo[b][1,4]dioxepine 1-Phenyl-1-cycloprppanecarboxylicacidRelated products of tetrahydrofuran
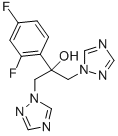
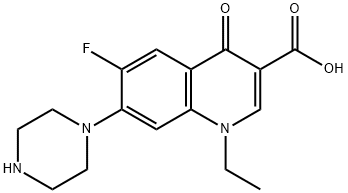

You may like
-
 Anti-THRB antibody produced in rabbit CASView Details
Anti-THRB antibody produced in rabbit CASView Details -
 3-(4-amino-1-oxoisoindolin-2-yl)-1-methylpiperidine-2,6-dione 98%View Details
3-(4-amino-1-oxoisoindolin-2-yl)-1-methylpiperidine-2,6-dione 98%View Details -
 614-19-7 98%View Details
614-19-7 98%View Details
614-19-7 -
 3112-85-4 Methyl phenyl sulfone 98%View Details
3112-85-4 Methyl phenyl sulfone 98%View Details
3112-85-4 -
 20677-73-0 (2,2-diethoxyethyl)methylamine 98%View Details
20677-73-0 (2,2-diethoxyethyl)methylamine 98%View Details
20677-73-0 -
 3-(4-(hydroxyamino)-1-oxoisoindolin-2-yl)piperidine-2,6-dione 98%View Details
3-(4-(hydroxyamino)-1-oxoisoindolin-2-yl)piperidine-2,6-dione 98%View Details -
 57381-49-4 2-bromo-4-chlorobenzonitrile 98%View Details
57381-49-4 2-bromo-4-chlorobenzonitrile 98%View Details
57381-49-4 -
 4,6-dichloropyrimidine-5-carbaldehyde 98%View Details
4,6-dichloropyrimidine-5-carbaldehyde 98%View Details
5305-40-8
