Propylene carbonate
Synonym(s):1,2-Propanediol cyclic carbonate;4-Methyl-1,3-dioxolan-2-one;4-Methyl-1,3-dioxolan-2-one, Propylene glycol carbonate, Carbonic acid propylene glycol ester;PC;Propylene carbonate
- CAS NO.:108-32-7
- Empirical Formula: C4H6O3
- Molecular Weight: 102.09
- MDL number: MFCD00005385
- EINECS: 203-572-1
- SAFETY DATA SHEET (SDS)
- Update Date: 2025-12-17 09:50:35
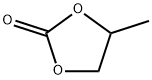
What is Propylene carbonate ?
Chemical properties
Propylene carbonate is a clear, colorless, mobile liquid, with a faint odor.
The Uses of Propylene carbonate
propylene carbonate is used in chemical reactions as a solvent, plasticizer, solubilizer, or dilutent. It is also used in the synthesis of solar cells as well as lithium ion batteries.
What are the applications of Application
Propylene carbonate is a polar, aprotic solvent used in electrolysis
Production Methods
Propylene carbonate may be prepared by the reaction of sodium bicarbonate with propylene chlorohydrin.
General Description
Propylene carbonate is a cyclic carbonate that is commonly used as a solvent and as a reactive intermediate in organic synthesis. It is being considered as a potential electrochemical solvent due to its low vapor pressure, high dielectric constant and high chemical stability.
Propylene carbonate can be synthesized from propylene oxide and CO2. Optically active form of propylene carbonate can be prepared from the reaction between CO2 and racemic epoxides. Decomposition of propylene carbonate on the graphite electrode in lithium batteries results in the formation of a lithium intercalated compound.
Flammability and Explosibility
Not classified
Pharmaceutical Applications
Propylene carbonate is used mainly as a solvent in oral and topical
pharmaceutical formulations.
In topical applications, propylene carbonate has been used in
combination with propylene glycol as a solvent for corticosteroids.
The corticosteroid is dissolved in the solvent mixture to yield microdroplets that can then be dispersed in petrolatum.Propylene
carbonate has been used as a dispensing solvent in topical
preparations.
Propylene carbonate has also been used in hard gelatin capsules
as a nonvolatile, stabilizing, liquid carrier. For formulations with a
low dosage of active drug, a uniform drug content may be obtained
by dissolving the drug in propylene carbonate and then spraying
this solution on to a solid carrier such as compressible sugar; the
sugar may then be filled into hard gelatin capsules
Propylene carbonate may additionally be used as a solvent, at
room and elevated temperatures, for many cellulose-based polymers
and plasticizers. Propylene carbonate is also used in cosmetics.
Safety
Propylene Carbonate is listed in The Design for the Environment (DfE) Safer Chemistry Program by the EPA as a Solvent category and indicated by the Green circle, meaning the chemical has been verified to be of low concern for human and environmental health based on experimental and modeled data.
In animal studies, propylene carbonate was found to cause tissue necrosis after parenteral administration.
(mouse, oral): 20.7 g/kg
(mouse, SC): 15.8 g/kg
(rat, oral): 29 g/kg
(rat, SC): 11.1 g/kg
Storage
Propylene carbonate and its aqueous solutions are stable but may
degrade in the presence of acids or bases, or upon heating;
Store in a well-closed container in a cool, dry place.
Purification Methods
It is manufactured by reaction of 1,2-propylene oxide with CO2 in the presence of a catalyst (quaternary ammonium halide). Contaminants include propylene oxide, carbon dioxide, 1,2-and 1,3-propanediols, allyl alcohol and ethylene carbonate. It can be purified by percolation through molecular sieves (Linde 5A, dried at 350o for 14hours under a stream of argon), followed by distillation under a vacuum. [Jasinski & Kirkland Anal Chem 39 163 1967.] It can be stored over molecular sieves under an inert gas atmosphere. When purified in this way it contains less than 2ppm of water. Activated alumina and dried CaO have also been used as drying agents prior to fractional distillation under reduced pressure. It has been dried with 3A molecular sieves and distilled under nitrogen in the presence of p-toluenesulfonic acid, then redistilled and the middle fraction collected. [Beilstein 19 III/IV 1564, 19/4 V 21.]
Incompatibilities
Propylene carbonate hydrolyzes rapidly in the presence of strong acids and bases, forming mainly propylene oxide and carbon dioxide. Propylene carbonate can also react with primary and secondary amines to yield carbamates.
Regulatory Status
Included in the FDA Inactive Ingredients Database (topical ointments). Included in the Canadian List of Acceptable Nonmedicinal Ingredients.
Properties of Propylene carbonate
| Melting point: | -55 °C (lit.) |
| Boiling point: | 240 °C (lit.) |
| Density | 1.204 g/mL at 25 °C (lit.) |
| vapor pressure | 0.13 mm Hg ( 20 °C) |
| refractive index | n |
| Flash point: | 270 °F |
| storage temp. | Store below +30°C. |
| solubility | 240g/l |
| form | Liquid |
| pka | 3.92[at 20 ℃] |
| Specific Gravity | 1.209 (20/4℃) |
| color | Clear |
| PH | 7.0 (200g/l, H2O, 20℃) |
| Odor | odorless |
| Relative polarity | 6 |
| explosive limit | 1.8-14.3%(V) |
| Water Solubility | 240 g/L (20 ºC) |
| λmax | λ: 235 nm Amax: 1.00 λ: 280 nm Amax: 0.50 λ: 300 nm Amax: 0.30 λ: 350 nm Amax: 0.05 λ: 375-400 nm Amax: 0.01 |
| BRN | 107913 |
| Dielectric constant | 64.4(Ambient) |
| Stability: | Stable. Incompatible with strong oxidizing agents, acids, bases, reducing agents. Protect from contact with moist air or water. |
| CAS DataBase Reference | 108-32-7(CAS DataBase Reference) |
| NIST Chemistry Reference | Propylene carbonate(108-32-7) |
| EPA Substance Registry System | Propylene carbonate (108-32-7) |
Safety information for Propylene carbonate
| Signal word | Warning |
| Pictogram(s) |
 Exclamation Mark Irritant GHS07 |
| GHS Hazard Statements |
H319:Serious eye damage/eye irritation |
| Precautionary Statement Codes |
P264:Wash hands thoroughly after handling. P264:Wash skin thouroughly after handling. P280:Wear protective gloves/protective clothing/eye protection/face protection. P305+P351+P338:IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. Continuerinsing. P337+P313:IF eye irritation persists: Get medical advice/attention. |
Computed Descriptors for Propylene carbonate
| InChIKey | RUOJZAUFBMNUDX-UHFFFAOYSA-N |
Propylene carbonate manufacturer
Jayas Ventures Private Limited
Arham Intermediates
Remark Chemical Enterprises
New Products
Indole Methyl Resin tert-butyl 9-methoxy-3-azaspiro[5.5]undecane-3-carboxylate Boc-His(Boc)-OH 2-CTC Resin 4-Chloro-7-tosy1-7Hpyrrolo[2,3-d]pyrimidine 5,7-Dibromo-1H-indole 2,5-dichloro-N-hydroxy-4,6-dimethylpyridine-3-carboximidamide 2,2-Dimethoxy-7-azaspiro[3.5]nonane hydrochloride 4-chloromethyl-5-methyl-1,3-dioxol-2-one (DMDO-Cl) R-2-BENZYLOXY PROPIONIC ACID 1,1’-CARBONYLDIIMIDAZOLE 1,1’-CARBONYLDI (1,2-4 TRIAZOLE) N-METHYL INDAZOLE-3-CARBOXYLIC ACID 4-((2-hydroxyethyl)thio)benzoic acid 1-(TERT-BUTOXYCARBONYL)-2-PYRROLIDINONE Methyl 6-methylnicotinate 3-Pyridineacrylic acid tert-Butyl carbazate TETRAHYDRO-2H-PYRAN-3-OL 2-((4-morpholinophenylamino) (methylthio) methylene) malononitrile 3-(4-morpholinophenylamino)-5-amino-1H-pyrazole-4-carbonitrile 2,4-dihydroxybenzaldehyde 1,3-Diethyl-1,3-Diphenylurea Methyl 2-methylquinoline-6-carboxylateRelated products of tetrahydrofuran
You may like
-
 Propylene Carbonate CASView Details
Propylene Carbonate CASView Details -
 Propylene Carbonate CASView Details
Propylene Carbonate CASView Details -
 Propylene Carbonate pure CAS 108-32-7View Details
Propylene Carbonate pure CAS 108-32-7View Details
108-32-7 -
 Shandong Lixing Propylene Carbonate Cas 108 32 7, Purity: 99.9%, Packaging Size: 250View Details
Shandong Lixing Propylene Carbonate Cas 108 32 7, Purity: 99.9%, Packaging Size: 250View Details
108-32-7 -
 CHINA Propylene Carbonate Cas 108 32 7, Grade: Technical, Purity: 99.5View Details
CHINA Propylene Carbonate Cas 108 32 7, Grade: Technical, Purity: 99.5View Details
108-32-7 -
 Propylene Carbonate Cas 108 32 7, For IndustriesView Details
Propylene Carbonate Cas 108 32 7, For IndustriesView Details
108-32-7 -
 Liquid Propylene CarbonateView Details
Liquid Propylene CarbonateView Details
108-32-7 -
 Propylene Carbonate 99%, For Industrial, Packaging Type: BottleView Details
Propylene Carbonate 99%, For Industrial, Packaging Type: BottleView Details
108-32-7
