Ethyl methyl carbonate
Synonym(s):Carbonic acid ethyl methyl ester;EMC;Methyl ethyl carbonate
- CAS NO.:623-53-0
- Empirical Formula: C4H8O3
- Molecular Weight: 104.1
- MDL number: MFCD00191398
- EINECS: 433-480-9
- SAFETY DATA SHEET (SDS)
- Update Date: 2025-01-27 09:38:02
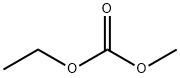
What is Ethyl methyl carbonate ?
Description
Ethyl methyl carbonate (EMC) is an asymmetric aliphatic organic carbonate. It is a colorless transparent liquid widely used as an excellent solvent for lithium-ion battery electrolytes. EMC increases the solubility of lithium ions improving the capacity density and power of batteries. This compound has excellent thermal conductivity and low electrical resistance, extending the working temperature range of electrolytes, improving lithium batteries' safety, and prolonging their service life. EMC is usually obtained by metal oxide-catalyzed transesterification over dimethyl carbonate and diethyl carbonate. High percentage yields are obtained if the reaction is carried out in liquid or vapor phase[1].
The Uses of Ethyl methyl carbonate
Non-aqueous solvent for Li-ion batteries
The Uses of Ethyl methyl carbonate
Alkyl carbonates find applications as solvents for lithium ion battery electrolytes and the use of high quality battery grade electrolytes having extremely low water (<10 ppm) and acid (<10 ppm) contents are critical for achieving high electrochemical performance.
The Uses of Ethyl methyl carbonate
EMC is majorly utilized as a co-solvent in the nonaqueous electrolyte. It enhances the energy density and discharge capacity of lithium-ion batteries.
General Description
This product has been enhanced for energy efficiency.
Advantages
Ethyl methyl carbonate enables the largest reversible capacity, best cycle stability, highest cycle Coulombic efficiency, lowest onset potential, smallest polarization, lowest self-discharge rate, and best rate performance from graphite compared to dimethyl carbonate and diethyl carbonate. Ethyl methyl carbonate forms a thinner layer of cathode electrolyte interface on the graphite surface with fewer Li–F and ROCO2Li species than with dimethyl carbonate and diethyl carbonate[2].
References
[1] Guido N. Rimondino , Fabio E. Malanca, Jesús A. Vila . “Atmospheric oxidation of ethyl methyl carbonate: Kinetics and reaction mechanism.” Journal of Photochemistry and Photobiology A-chemistry 444 (2023): Article 114994.
[2] Yao Wang. “Unlocking the True Capability of Graphite-Based Dual-Ion Batteries with Ethyl Methyl Carbonate Electrolyte.” ACS Applied Energy Materials 2 10 (2019): 7512–7517.
Properties of Ethyl methyl carbonate
| Melting point: | -14.5℃ |
| Boiling point: | 107 °C |
| Density | 1.006 g/mL at 25 °C |
| vapor pressure | 43hPa at 25℃ |
| refractive index | n20/D 1.378 |
| Flash point: | 23°C |
| storage temp. | 2-8°C |
| form | clear liquid |
| color | Colorless to Almost colorless |
| Water Solubility | 46.8-47.1g/L at 20℃ |
| InChI | InChI=1S/C4H8O3/c1-3-7-4(5)6-2/h3H2,1-2H3 |
| CAS DataBase Reference | 623-53-0(CAS DataBase Reference) |
| NIST Chemistry Reference | C2H5OCOOCH3(623-53-0) |
| EPA Substance Registry System | Carbonic acid, ethyl methyl ester (623-53-0) |
Safety information for Ethyl methyl carbonate
| Signal word | Danger |
| Pictogram(s) |
 Flame Flammables GHS02 |
| GHS Hazard Statements |
H225:Flammable liquids |
| Precautionary Statement Codes |
P210:Keep away from heat/sparks/open flames/hot surfaces. — No smoking. |
Computed Descriptors for Ethyl methyl carbonate
| InChIKey | JBTWLSYIZRCDFO-UHFFFAOYSA-N |
| SMILES | C(OC)(=O)OCC |
New Products
1-Amino-1-cyclohexanecarboxylic acid N-Boc-2-bromoethylamine 2-Hydroxy-N-methylacetamide ELECTROLYTIC IRON POWDER 1-Aminocyclobutanecarboxylic acid 1-(2-Ethoxyethyl)-2-(piperidin-4-yl)-1H-benzo[d]imidazole hydrochloride Decanonitrile tert-butyl 4-(1H-benzo[d]iMidazol-2-yl)piperidine-1-carboxylate N,N'-diallyl-1,3-diaminopropanedihydrochloride 3-Iodo-6-nitroindazole Cyclopropanamine hydrochloride 4-methoxybenzylbromide (3,5-dibromophenyl)methanamine 2-chloro-4-methyl-5-nitro pyridine 6-bromo-5-cyano quinoline 5-Bromo-2-chloro-3-methoxypyridine 2,5-dibromopyridine terephthalonitrile 5-Bromo-4-chloro-2(1h)-pyridinone, 5-Fluoro-2-Oxindole N-Vinylformamide 2-ethyl-6-methyl-3-hydroxypyridine succinate Ste-Glu-AEEA-AEEA-OSU Chloro UracilRelated products of tetrahydrofuran
You may like
-
 Ethyl methyl carbonate 95% CAS 623-53-0View Details
Ethyl methyl carbonate 95% CAS 623-53-0View Details
623-53-0 -
 Ethyl Methyl Carbonate CAS 623-53-0View Details
Ethyl Methyl Carbonate CAS 623-53-0View Details
623-53-0 -
 Ethyl methyl carbonate CAS 623-53-0View Details
Ethyl methyl carbonate CAS 623-53-0View Details
623-53-0 -
 Ethyl methyl carbonate CAS 623-53-0View Details
Ethyl methyl carbonate CAS 623-53-0View Details
623-53-0 -
 Ethyl methyl carbonate CAS 623-53-0View Details
Ethyl methyl carbonate CAS 623-53-0View Details
623-53-0 -
 623-53-0 BENZYL CYNO ETHYL METHYL CARBONATE 99%View Details
623-53-0 BENZYL CYNO ETHYL METHYL CARBONATE 99%View Details
623-53-0 -
 Ethyl methyl carbonate 99%View Details
Ethyl methyl carbonate 99%View Details -
 6-bromo-4-chloro-N-methylquinolin-3-amine 98%View Details
6-bromo-4-chloro-N-methylquinolin-3-amine 98%View Details