Procymidone
- CAS NO.:32809-16-8
- Empirical Formula: C13H11Cl2NO2
- Molecular Weight: 284.14
- MDL number: MFCD00078740
- EINECS: 251-233-1
- SAFETY DATA SHEET (SDS)
- Update Date: 2025-01-27 09:38:02
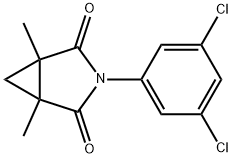
What is Procymidone?
The Uses of Procymidone
Procymidone is a systemic fungcide with both protective and curative activities used to control plant diseases such as fruit rots, grey mould on top fruits, vines and vegetables, and Sclerotinia rot of kidney beans and vegetable crops.
The Uses of Procymidone
Systemic agricultural fungicide.
Definition
ChEBI: An azabicycloalkane that is 1,5-dimethyl-3-azabicyclo[3.1.0]hexane-2,4-dione in which the amino hydrogen is replaced by a 3,5-dichlorophenyl group. A fungicide widely used in horticulture as a seed dressing, pre-harvest spray or post-harvest dip for the co trol of various diseases.
Metabolic pathway
The fungicides, chlozolinate, vinclozolin, and procymidone, are added to wine after fermentation and the degradation products are isolated and identified. Chlozolinate undergoes a rapid hydrolytic loss of the ethoxycarbonyl substituent to give an oxazolidine that further undergoes hydrolytic cleavage to give 3' ,5' -dichloro-2-hydroxypropanilide. The oxazolidine ring of vinclozolin undergoes a similar hydrolysis reaction to give the corresponding anilide, 3' ,5'-dichloro-2-hydroxy-2-methylbut-3-eneanilide. Both of these anilides are stable in wine for 150 days. A different degradation behavior is observed with procymidone and leads to the formation of 3,5- dichloroaniline, which, in turn, breaks down in wine.
Degradation
Procymidone (1) was stable in acidic conditions (pH 2) but hydrolysed
rapidly in buffer solutions (pH 6 to 10) and in natural river and sea water
at 15, 30 and 45 °C with DTm values from 30 min to 8 days. Degradation
occurred mainly via cleavage of the cyclic imide linkage in alkaline
conditions and via cleavage of the subsequent amide linkage in acidic
conditions (Mikami and Miyamoto, 1981) to yield the intermediate acid
[2-( 3,5-dichlorophenylcarbamoyl)- 1,2 -dimet hylcyclopropanecarboxylic
acid (2) and 1,2-dimethylcyclopropane-1,2-dicarboxylica cid (3) and 3,5-
dichloroaniline (4) as terminal products (Villedieu et al., 1994,1995). Pirisi
et al. (1986) and Cabras et al. (1984) reported the formation of compound 4
and other polar decomposition products in wine during the fermentation
process.
Photodegradation of procymidone was investigated in various
solutions after exposure to sunlight. Major degradation pathways were
cleavage of the cyclic imide and the subsequent cleavage of the amide
linkage to yield compounds 3 and 4 (Mikami and Miyamoto, 1981).
Properties of Procymidone
| Melting point: | 166-167°C |
| Boiling point: | 477.9±35.0 °C(Predicted) |
| Density | d25 1.42-1.46 |
| vapor pressure | 1.8 x 10-2 Pa (25 °C) |
| refractive index | 1.6100 (estimate) |
| Flash point: | >100 °C |
| storage temp. | Sealed in dry,Room Temperature |
| solubility | Chloroform (Slightly), Ethyl Acetate (Slightly) |
| form | neat |
| Water Solubility | 4.5 mg l-1 (25 °C) |
| pka | -2.67±0.60(Predicted) |
| color | Off-White to Pale Beige |
| BRN | 1539058 |
| CAS DataBase Reference | 32809-16-8(CAS DataBase Reference) |
| NIST Chemistry Reference | 3-Azabicyclo[3.1.0]hexane-2,4-dione, 3-(3,5-dichlorophenyl)-1,5-dimethyl-(32809-16-8) |
| EPA Substance Registry System | Procymidone (32809-16-8) |
Safety information for Procymidone
Computed Descriptors for Procymidone
| InChIKey | QXJKBPAVAHBARF-UHFFFAOYSA-N |
New Products
(R)-1-Boc-3-hydroxypyrrolidine Methyl (R)-1-Boc-4,4-difluoropyrrolidine-2-carboxylate 2,2-Difluoropropylamine hydrochloride tert-butyl 3-bromoazetidine-1-carboxylate DIFLUOROACETIC ANHYDRIDE 2,2-Difluoropropionic acid Diallylamine, 99% Calcium hydroxide, 95% Aluminum oxide, basic 2-Bromophenylacetonitrile, 97% L-tert-Leucine,97% N-Hydroxy-2-methylpropanimidamide 4-(3,4-Dichlorophenyl)-3,4-Dihydro-N-Methyl-1-(2H)-Naphthalenimine (Schiff Base) 2-AMINO-3,5-DIBROMO BENZALDEHYDE [ADBA] L-Glutamic Acid Dimethyl Ester Hcl 10-Methoxy-5H-dibenz[b,f]azepine 5-Cyanophthalide N, N-Carbonyldiimidazole (CDI) Dibenzoyl Peroxide Titanium Dioxide 2-(Methylthio) Benzonitrile Sodium Acetate Anhydrous Allopurinol 1,5-DibromopentaneRelated products of tetrahydrofuran




You may like
-
 Procymidone CASView Details
Procymidone CASView Details -
![Cis-2-(Bromomethyl)-2-(2,4-Dichlorophenyl)-1,3-Dioxolane-4-Ylmethyl Benzoate [CBB] 61397-56-6 99%](https://img.chemicalbook.in//Content/image/CP5.jpg) Cis-2-(Bromomethyl)-2-(2,4-Dichlorophenyl)-1,3-Dioxolane-4-Ylmethyl Benzoate [CBB] 61397-56-6 99%View Details
Cis-2-(Bromomethyl)-2-(2,4-Dichlorophenyl)-1,3-Dioxolane-4-Ylmethyl Benzoate [CBB] 61397-56-6 99%View Details
61397-56-6 -
 287930-77-2 / 142569-70-8 99%View Details
287930-77-2 / 142569-70-8 99%View Details
287930-77-2 / 142569-70-8 -
 Ethyl-2-Chloroacetoacetate 609-15-4View Details
Ethyl-2-Chloroacetoacetate 609-15-4View Details
609-15-4 -
 CIS- BROMO BENZOATEView Details
CIS- BROMO BENZOATEView Details
61397-56-6 -
 609-15-4View Details
609-15-4View Details
609-15-4 -
![1-(6-Methylpyridin-3-Yl)-2-[4-(Methylsulfonyl)Phenyl]Ethanone [Ketosulfone] 99%](https://img.chemicalbook.in//Content/image/CP5.jpg) 1-(6-Methylpyridin-3-Yl)-2-[4-(Methylsulfonyl)Phenyl]Ethanone [Ketosulfone] 99%View Details
1-(6-Methylpyridin-3-Yl)-2-[4-(Methylsulfonyl)Phenyl]Ethanone [Ketosulfone] 99%View Details
221615-75-4 -
 27143-07-3View Details
27143-07-3View Details
27143-07-3
