Hexane
Synonym(s):Hexane;Hexanes;Hexane in dimethyl sulfoxide;Hexane solution;n-Hexane
- CAS NO.:110-54-3
- Empirical Formula: C6H14
- Molecular Weight: 86.18
- MDL number: MFCD00009520
- EINECS: 203-777-6
- SAFETY DATA SHEET (SDS)
- Update Date: 2025-12-17 09:49:25
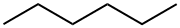
What is Hexane?
Description
The hexane that are commonly used in organic chemistry are a mixture of various six carbon alkanes, not just n-hexane. The lower toxicity and flammability of heptane has led to a slow shift towards heptane in favor of hexane.
Chemical properties
n-Hexane is a highly flammable liquid typically derived from crude oil and widely used as an industrial solvent, including in adhesive bandage manufacturing. It is highly toxic to both animals and humans, causing adverse health effects such as nausea, skin irritation, dizziness, limb numbness, CNS depression, vertigo, and respiratory tract irritation. Occupational exposure has been linked to motor polyneuropathy in workers, with long-term exposure—such as in glue sniffing—associated with degenerative damage to axons and nerve terminals.
Chemical properties
n-Hexane is a highly flammable, colorless, and volatile liquid with a gasoline-like odor. Its odor threshold is 0.0064 mg/L in water and 230,875 mg/m3 in air.
The Uses of Hexane
To a solution of the SM (1.12 g, 6.66 mmol) in dry ether (19 mL) at 0 C was added dropwise PBr3 (0.626 mL, 6.66 mmol). The ice bath was removed and the reaction was stirred for 3 h. H2O was carefully added to the mixture and the layers were separated. The org layer was washed with brine, dried (MgSO4), and concentrated in vacuo to provide the product. [1.49 g, 97%]
General Description
A clear, colorless liquid with a petroleum-like odor and a flash point of -9°F. It is less dense than water and insoluble in water, with vapors heavier than air. It is used as a solvent, paint thinner, and chemical reaction medium.
Reactivity Profile
n-Hexane may be sensitive to light and prolonged heat exposure. It reacts vigorously with oxidizing materials, including liquid chlorine, concentrated oxygen, sodium hypochlorite, and calcium hypochlorite, and is incompatible with dinitrogen tetroxide. Additionally, it can degrade certain forms of plastics, rubber, and coatings.
Health Hazard
n-Hexane is a respiratory irritant and narcotic at high concentrations, with acute toxicity greater than n-pentane. Short-term exposure can cause convulsions and death in animals, while humans may experience hallucinations, distorted vision, headache, dizziness, nausea, and eye/throat irritation after 10 minutes at around 5000 ppm. Chronic exposure may lead to polyneuritis. Its metabolites, 2,5-hexanedione and 5-hydroxy-2-hexanone, are shared with methyl butyl ketone, but the neurotoxic metabolite 2,5-hexanedione is produced less extensively. Continuous exposure to 250 ppm induces neurotoxic effects in animals, and occupational exposure to 500 ppm may cause polyneuropathy. Additionally, n-hexane vapor has shown reproductive toxicity in rats and mice.
Potential Exposure
n-Hexane is an industrial chemical used as an emulsifier in the production of plastics and resins, as a solvent—particularly for extracting edible fats and oils—as a laboratory reagent, and as a filling fluid in low-temperature thermometers. Technical and commercial grades typically contain 45–85% n-hexane, along with cyclopentane, isohexane, and 1–6% benzene.
Source
n-Hexane is a component of gasoline, accounting for 1.2% of hydrocarbons in diesel engine exhaust (Verschueren, 1983). In unleaded gasoline with ethanol replacing methyl tert-butyl ether, hexane concentrations in headspace vapor were 4.31 wt% for regular grade, 3.74–4.8 wt% for midgrade, and 2.3 wt% for premium grade (Harley et al., 2000). Tailpipe emission rates from gasoline vehicles were 1.82 mg/km with catalytic converters and 268 mg/km without (Schauer et al., 2002).
Purification Methods
n-Hexane can be purified by washing with chlorosulfonic acid or 35% fuming sulfuric acid instead of concentrated H?SO?, followed by drying and distillation over sodium hydride. Unsaturated impurities are removed by shaking with nitrating acid (e.g., 58% H?SO? + 25% HNO?), then washing the hydrocarbon layer with concentrated H?SO? and water, drying, and distilling over sodium or n-butyl lithium. Alternative methods include distillation under nitrogen from sodium benzophenone ketyl in tetraglyme, or passage through a silica gel column prior to distillation. As a flammable liquid and potential neurotoxin, rapid purification involves discarding the initial distillate and storing over 4A molecular sieves.
Properties of Hexane
| Melting point: | -95 °C |
| Boiling point: | 68.95 °C(lit.) |
| Density | 0.659 g/mL at 25 °C(lit.) |
| vapor density | 3.5 (vs air) |
| vapor pressure | 40 mm Hg ( 20 °C) |
| refractive index | n |
| Flash point: | 30 °F |
| storage temp. | Store at +5°C to +30°C. |
| solubility | Very soluble in ethanol, ethyl ether and chloroform. |
| form | Liquid |
| appearance | Colorless liquid |
| pka | >14 (Schwarzenbach et al., 1993) |
| Specific Gravity | 0.660 (20/4℃) |
| color | Colorless |
| Odor | Mild gasoline-like odor detectable at 65 to 248 ppm |
| Relative polarity | 0.009 |
| explosive limit | 1.0-8.1%(V) |
| Odor Threshold | 1.5ppm |
| Water Solubility | insoluble |
| λmax | λ: 200 nm Amax: ≤0.70 λ: 225 nm Amax: ≤0.10 λ: 250 nm Amax: ≤0.01 |
| Merck | 14,4694 |
| BRN | 1730733 |
| Henry's Law Constant | 0.238, 0.413, 0.883, 0.768, and 1.56 at 10, 15, 20, 25, and 30 °C, respectively (EPICS, Ashworth
et al., 1988) |
| Exposure limits | TLV-TWA 50 ppm (~175 mg/m3) (ACGIH),
500 ppm (~1750 mg/m3) (OSHA); IDLH
5000 ppm (NIOSH). |
| Dielectric constant | 2.0(-90℃) |
| Stability: | Stable. Incompatible with oxidizing agents, chlorine, fluorine, magnesium perchlorate. Highly flammable. Readily forms explosive mixtures with air. Note low flash point. |
| CAS DataBase Reference | 110-54-3(CAS DataBase Reference) |
| NIST Chemistry Reference | Hexane(110-54-3) |
| EPA Substance Registry System | Hexane (110-54-3) |
Safety information for Hexane
| Signal word | Danger |
| Pictogram(s) |
 Flame Flammables GHS02  Exclamation Mark Irritant GHS07  Health Hazard GHS08  Environment GHS09 |
| GHS Hazard Statements |
H225:Flammable liquids H304:Aspiration hazard H315:Skin corrosion/irritation H336:Specific target organ toxicity,single exposure; Narcotic effects H373:Specific target organ toxicity, repeated exposure H411:Hazardous to the aquatic environment, long-term hazard |
| Precautionary Statement Codes |
P201:Obtain special instructions before use. P210:Keep away from heat/sparks/open flames/hot surfaces. — No smoking. P273:Avoid release to the environment. P331:Do NOT induce vomiting. P301+P310:IF SWALLOWED: Immediately call a POISON CENTER or doctor/physician. P303+P361+P353:IF ON SKIN (or hair): Remove/Take off Immediately all contaminated clothing. Rinse SKIN with water/shower. |
Computed Descriptors for Hexane
| InChIKey | VLKZOEOYAKHREP-UHFFFAOYSA-N |
Hexane manufacturer
New Products
4,4-Difluoropiperidine hydrochloride tert-butyl 9-methoxy-3-azaspiro[5.5]undecane-3-carboxylate Indole Methyl Resin N-Isopropylurea N,N-Dicyclohexylcarbodiimide(DCC) MELDRUMS ACID 5-METHYLISOXAZOLE-4-CARBOXYLIC ACID Magnessium Bis glycinate Zinc ascorbate 1-bromo-2-butyne 2-acetamidophenol 9(10H)-anthracenone Erythrosin B, 4-Piperidinopiperidine 2-((4-morpholinophenylamino) (methylthio) methylene) malononitrile 2,4-dihydroxybenzaldehyde 3-(4-morpholinophenylamino)-5-amino-1H-pyrazole-4-carbonitrile Methyl 2-methylquinoline-6-carboxylate 2,6-dichloro-4-nitropyridine 4-Bromo-2-chlorobenzonitrile 2-(benzylamino)acetic acid hydrochloride 4-(tert-Butoxycarbonylamino)but- 2-ynoic acid 3,4-dihydro-2H-benzo[b][1,4]dioxepine 1-Phenyl-1-cycloprppanecarboxylicacidRelated products of tetrahydrofuran



You may like
-
 N-Hexane 99%View Details
N-Hexane 99%View Details -
 n-Hexane CAS 110-54-3View Details
n-Hexane CAS 110-54-3View Details
110-54-3 -
 n-Hexane CAS 110-54-3View Details
n-Hexane CAS 110-54-3View Details
110-54-3 -
 n-Hexane CAS 110-54-3View Details
n-Hexane CAS 110-54-3View Details
110-54-3 -
 n-Hexane CAS 110-54-3View Details
n-Hexane CAS 110-54-3View Details
110-54-3 -
 n-Hexane CAS 110-54-3View Details
n-Hexane CAS 110-54-3View Details
110-54-3 -
 n-Hexane CAS 110-54-3View Details
n-Hexane CAS 110-54-3View Details
110-54-3 -
 Hexane, HPLC Grade 99%View Details
Hexane, HPLC Grade 99%View Details
