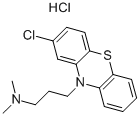
Prochlorperazine
- CAS NO.:58-38-8
- Empirical Formula: C20H24ClN3S
- Molecular Weight: 373.94
- MDL number: MFCD00056797
- EINECS: 200-379-4
- SAFETY DATA SHEET (SDS)
- Update Date: 2023-06-08 13:53:37
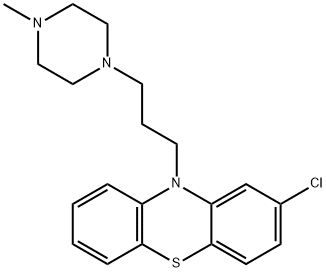
What is Prochlorperazine?
Absorption
Following oral administration, prochlorperazine is reported to be well absorbed from the gastrointestinal tract. The onset of pharmacological action is about 30 to 40 minutes following oral administration and 10 to 20 minutes following intramuscular administration. The duration of action for all routes is about 3 to 4 hours. Following oral administration in healthy volunteers, the mean oral bioavailability was about 12.5%. In these patients, the time to reach the peak plasma concentrations was about 5 hours. Repeated oral dosing resulted in an accumulation of prochlorperazine and its metabolite. Following multiple twice daily dosing, the steady state of prochlorperazine was reached by 7 days.
Toxicity
LD50 and Overdose
Oral LD50 in rats is 750 mg/kg. Intraperitoneal and subcutaneous LD50 in mice are 191 mg/kg and 320 mg/kg, respectively. In placebo-controlled trials, there were increased incidences of mortality in elderly patients with dementia-related psychosis receiving antipsychotic medications. The risk of death in drug-treated patients was about 1.6 to 1.7 times that of placebo-treated patients. Deaths were largely resulting from cardiovascular, such as heart failure and sudden death, or infectious, such as pneumonia, conditions. Due to its antagonist action on dopamine receptors, prochlorperazine is associated with a risk for developing extrapyramidal symptoms such as tardive dyskinesia, which is a syndrome consisting of potentially irreversible, involuntary, dyskinetic movements. This risk is also conferred on other antipsychotic agents that block dopamine receptors. It is proposed that increased duration of the drug treatment is likely thus increased total cumulative dose of antipsychotic drugs administered to the patient leads to increased risk for developing the syndrome and the likelihood that it will become irreversible. As with other antipsychotic agents, prochlorperazine is associated with a risk for causing neuroleptic malignant syndrome (NMS), which is a potentially fatal symptom complex, which is manifested as hyperpyrexia, muscle rigidity, altered mental status and evidence of autonomic instability.
There is no known antidote for prochlorperazine thus overdose treatment should be supportive and symptomatic. Overdose of prochlorperazine may produce dystonic reactions that involve extrapyramidal mechanism. To reduce these symptoms, antiparkinsonism drugs, barbiturates, or diphenhydramine may be used. Symptoms of central nervous system depression, such as somnolence or coma, may also be observed. Amphetamine, destroamphetamine, or caffeine and sodium benzoate may be used to induce stimulatory effects. In contrast, agitation and restlessness may also be seen in case of overdose. Other possible manifestations include convulsions, EKG changes and cardiac arrhythmias, fever, and autonomic reactions such as hypotension, dry mouth and ileus. Hypotension can be responded with the standard measures for managing circulatory shock.
Nonclinical Toxicology
In a rat developmental or reproductive toxicity study, abnormalities in both the reproductive measures and neurobehavioral testing were observed following administration of 25 mg/kg of prochlorperazine.
Use in specific populations
As the use of antipsychotic agents during the third trimester of pregnancy is associated with a risk for extrapyramidal and/or withdrawal symptoms following delivery, the use of prochlorperazine in pregnant patients is generally not recommended and it should be limited after careful consideration of the potential benefit of drug therapy justifying the potential risk to the fetus. Caution should be exercised when prochlorperazine is administered to a nursing mother. While lower doses of prochlorperazine is reported to be safe for elderly patients, caution is still advised, especially those with higher susceptibility to hypotension and neuromuscular reactions.
The Uses of Prochlorperazine
Prochlorperazine is antiemetic, antipsychotic; used in treatment of vertigo.
The Uses of Prochlorperazine
antiemetic, antipsychotic, treatment of vertigo
Background
Prochlorperazine, also known as compazine, is a piperazine phenothiazine and first-generation antipsychotic drug that is used for the treatment of severe nausea and vomiting, as well as short-term management of psychotic disorders such as generalized non-psychotic anxiety and schizophrenia. It mainly works by depressing the chemoreceptor trigger zone and blocking D2 dopamine receptors in the brain. It was shown to also block histaminergic, cholinergic and noradrenergic receptors. Prochlorperazine was first developed in the 1950s and was first approved by the FDA in 1956. Although newer antiemetic agents such as 5-HT3 antagonists are more heavily promoted, prochlorperazine is still widely used in nausea and vomiting.
Indications
Indicated for the symptomatic treatment of severe nausea and vomiting.
Indicated for the management of manifestations of psychotic disorders, such as schizophrenia and generalized non-psychotic anxiety. The use of prochlorperazine for the management of generalized non-psychotic anxiety is typically not a first-line therapy and should be limited to doses of less than 20 mg per day or for shorter than 12 weeks.
Off-label uses include use in emergency settings for adult and pediatric migraines. The American Headache Society recommends the use of prochlorperazine as the first-line medication in this setting. In pediatric migraines, a non-steroidal anti-inflammatory agent is often used in combination with dopamine antagonist.
Definition
ChEBI: A member of the class of phenothiazines that is 10H-phenothiazine having a chloro substituent at the 2-position and a 3-(4-methylpiperazin-1-yl)propyl group at the N-10 position.
brand name
Compazine (GlaxoSmithKline).
Pharmacokinetics
Prochlorperazine is an antipsychotic agent that works to promote postsynaptic inhibition of dopaminergic neurons. It also exerts its anti-emetic actions via anti-dopaminergic effects, where it displays similar efficacy as ondansteron, a 5HT-3 receptor antagonist and anti-emetic, in preventing delayed nausea and vomiting. Prochlorperazine was shown to inhibit histaminergic, cholinergic and alpha-1 adrenergic receptors. The blockade of alpha-1 adrenergic receptors may result in sedation, muscle relaxation, and hypotension. It displays anti-anxiety effects as well. Compared to other phenothiazine derivatives, prochlorperazine is less sedating and has a weak propensity for causing hypotension or potentiating the effects of CNS depressants and anesthetics. Other than its primary action on D2 receptors, one study showed that prochlorperazine may inhibit the P2X7 receptor in human macrophages, leading to inhibition of calcium ion influx.
Clinical Use
Nausea and vomiting
Labyrinthine disorders
Psychoses
Severe anxiety
Safety Profile
Poison by ingestion, subcutaneous, intravenous, and intraperitoneal routes. Experimental teratogenic and reproductive effects. Human systemic effects by ingestion: headache, blood pressure elevation. Implicated in aplastic anemia. When heated to decomposition it emits very toxic fumes of SOx, NOx, and Cl-.
Veterinary Drugs and Treatments
Prochlorperazine as a single agent is used in dogs and cats as an antiemetic. The only approved products for animals are combination products containing prochlorperazine, isopropamide, with or without neomycin (Darbazine?, Neo-Darbazine?—SKB Labs) which are no longer marketed in the USA. The approved indications for these products include: vomiting, non-specific gastroenteritis, drug induced diarrhea, infectious diarrhea, spastic colitis, and motion sickness in dogs and cats (injectable product only).
Drug interactions
Potentially hazardous interactions with other drugs
Anaesthetics: enhanced hypotensive effect.
Analgesics: increased risk of convulsions with
tramadol; enhanced hypotensive and sedative
effects with opioids; increased risk of ventricular
arrhythmias with methadone.
Anti-arrhythmics increased risk of ventricular
arrhythmias with anti-arrhythmics that prolong
the QT interval, e.g. procainamide, disopyramide,
dronedarone and amiodarone - avoid with
amiodarone and dronedarone.
Antibacterials: increased risk of ventricular
arrhythmias with delamanid and moxifloxacin -
avoid.
Antidepressants: increase concentrations and
additive antimuscarinic effects, notably with
tricyclics; increased risk of ventricular arrhythmias
with citalopram and escitalopram - avoid; increased
risk of convulsions with vortioxetine.
Antiepileptics: antagonised (convulsive threshold
lowered).
Antimalarials: avoid with artemether/lumefantrine
and piperaquine with artenimol.
Antipsychotics: increased risk of ventricular
arrhythmias with droperidol and pimozide - avoid;
increased risk of ventricular arrhythmias with
risperidone.
Antivirals: concentration possibly increased with
ritonavir; increased risk of ventricular arrhythmias
with saquinavir - avoid.
Anxiolytics and hypnotics: increased sedative effects.
Atomoxetine: increased risk of ventricular
arrhythmias.
Beta-blockers: enhanced hypotensive effect;
increased risk of ventricular arrhythmias with sotalol.
Cytotoxics: increased risk of ventricular arrhythmias
with arsenic trioxide.
Desferrioxamine: avoid concomitant use.
Diuretics: enhanced hypotensive effect.
Lithium: increased risk of extrapyramidal side effects
and possibly neurotoxicity.
Pentamidine: increased risk of ventricular
arrhythmias.
Metabolism
Prochlorperazine undergoes hepatic metabolism involving oxidation, hydroxylation, demethylation, sulfoxide formation and conjugation with glucuronic acid. The oxidation reaction is mediated by CYP2D6. N-desmethyl prochlorperazine was detected in the plasma, as well as prochlorperazine sulfoxide, prochlorperazine 7-hydroxide and prochlorperazine sulfoxide 4'-N-oxide, following oral and buccal administration. Prochlorperazine may enter the enterohepatic circulation.
Metabolism
Prochlorperazine undergoes extensive first pass metabolism in the gut wall. It is also extensively metabolised in the liver and is excreted in the urine and bile. The metabolites are inactive.
Properties of Prochlorperazine
| Melting point: | 228 °C |
| Boiling point: | 260-275 °C(Press: 2 Torr) |
| Density | 1.1679 (rough estimate) |
| refractive index | 1.6000 (estimate) |
| storage temp. | Refrigerator |
| solubility | Chloroform (Slightly), Ethyl Acetate (Slightly), Methanol (Slightly) |
| pka | pKa 8.1(H2O,t =24±1,I undefined) (Uncertain) |
| form | Solid |
| color | White to Yellow Oil to Waxy |
| Water Solubility | 14.96mg/L(24 ºC) |
| CAS DataBase Reference | 58-38-8(CAS DataBase Reference) |
| NIST Chemistry Reference | Prochlorperazine(58-38-8) |
Safety information for Prochlorperazine
Computed Descriptors for Prochlorperazine
Prochlorperazine manufacturer
Ralington Pharma
Biophore India Pharmaceuticals Pvt Ltd
New Products
4-Fluorophenylacetic acid 4-Methylphenylacetic acid N-Boc-D-alaninol N-BOC-D/L-ALANINOL Tert-butyl bis(2-chloroethyl)carbamate 3-Morpholino-1-(4-nitrophenyl)-5,6-dihydropyridin- 2(1H)-one Furan-2,5-Dicarboxylic Acid Tropic acid S-2-CHLORO PROPIONIC ACID ETHYL ISOCYANOACETATE 2-Bromo-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate (6-METHYL-[1,3]DITHIOLO[4,5-b]QUINOXALIN-2-ONE INDAZOLE-3-CARBOXYLIC ACID 4-IODO BENZOIC ACID (2-Hydroxyphenyl)acetonitrile 4-Bromopyrazole 5,6-Dimethoxyindanone 2-(Cyanocyclohexyl)acetic acid 4-methoxy-3,5-dinitropyridine 2-aminopropyl benzoate hydrochloride 1-(4-(aminomethyl)benzyl)urea hydrochloride diethyl 2-(2-((tertbutoxycarbonyl)amino) ethyl)malonate tert-butyl 4- (ureidomethyl)benzylcarbamate Ethyl-2-chloro((4-methoxyphenyl)hydrazono)acetateRelated products of tetrahydrofuran

You may like
-
 58-38-8 Prochlorperazine 98%View Details
58-38-8 Prochlorperazine 98%View Details
58-38-8 -
 58-38-8 98%View Details
58-38-8 98%View Details
58-38-8 -
 Prochlorperazine for system suitability CAS 58-38-8View Details
Prochlorperazine for system suitability CAS 58-38-8View Details
58-38-8 -
 1975-50-4 98%View Details
1975-50-4 98%View Details
1975-50-4 -
 2-HYDROXY BENZYL ALCOHOL 98%View Details
2-HYDROXY BENZYL ALCOHOL 98%View Details
90-01-7 -
 2-Chloro-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate 221615-75-4 98%View Details
2-Chloro-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate 221615-75-4 98%View Details
221615-75-4 -
 14714-50-2 (2-Hydroxyphenyl)acetonitrile 98+View Details
14714-50-2 (2-Hydroxyphenyl)acetonitrile 98+View Details
14714-50-2 -
 118753-70-1 98+View Details
118753-70-1 98+View Details
118753-70-1