Xylazine
Synonym(s):N-(2,6-Dimethylphenyl)-5,6-dihydro-4H-1,3-thiazin-2-amine;2-(2,6-Dimethylphenylamino)-5,6-dihydro-4H-thiazine;Xylazine
- CAS NO.:7361-61-7
- Empirical Formula: C12H16N2S
- Molecular Weight: 220.33
- MDL number: MFCD00057908
- EINECS: 230-902-1
- SAFETY DATA SHEET (SDS)
- Update Date: 2024-05-30 19:23:35
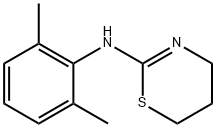
What is Xylazine?
Description
Xylazine is an agonist of α2-adrenergic receptors (Ki = 194 nM). It is an analog of clonidine, an α2-adrenergic receptor agonist used to reduce blood pressure. Xylazine is used for sedation, anesthesia, and analgesia in non-human mammals. This product is also available as an analytical reference standard .
Originator
Xylazine,Bayer
The Uses of Xylazine
Xylazine is an α2 class of adrenergic receptor agonist. Xylazine is a clonidine analoque that acts on presynaptic and postsynaptic receptors as an a 2-adrenergic agonist.
The Uses of Xylazine
Antinociceptive;Alpha-2 adrenergic agonist
What are the applications of Application
Xylazine is an α2 class of adrenergic receptor agonist
Definition
ChEBI: Xylazine is a methyl benzene that is 1,3-dimethylbenzene which is substituted by a 5,6-dihydro-4H-1,3-thiazin-2-ylnitrilo group at position 2. It is an alpha2 adrenergic receptor agonist and frequently used in veterinary medicine as an emetic and sedative with analgesic and muscle relaxant properties. It has a role as an emetic, an alpha-adrenergic agonist, a sedative, a muscle relaxant and an analgesic. It is a methylbenzene, a 1,3-thiazine and a secondary amino compound. It is a conjugate base of a xylazine(1+).
Manufacturing Process
2,6-Dimethylphenyl isothiocyanate, 31.0 g (0.2 mole), prepared from 2,6- dimethylaniline with thiophosgene, were added dropwise during 15 min to a well-stirred suspension of 15.0 g (0.2 mole) of 3-aminopropanol-1 in 100 ml of ether. The ether started to boil. Stirring under reflux was continued for 30 min, and the ether was then distilled off. The residue was treated with 100 ml of concentrated hydrochloric acid and boiled under reflux for 30 min. After cooling, it was diluted with water, filtered free from impurities, and the base was precipitated by the addition of concentrated sodium hydroxide solution. When recrystallized from benzene-ligroin, the resulting compound 2-(2,6- dimethyl-phenylamino)-4H-5,6-dihydro-1,3-thiazine, melting point 140-142°C (yield 90% of the theoretical).
Therapeutic Function
Analgesic, Anesthetic
Origin
Xylazine was discovered as an antihypertensive agent in 1962 by Farbenfabriken Bayer in Leverkusen, Germany. Accounts of the actions and uses of xylazine in animals were reported as early as the late 1960s and early 1970s. Results from early human clinical studies confirmed that xylazine has several central nervous system depressant effects.
General Description
Xylazine is soluble in methanol (50 mg/ml), yielding a clear, colorless solution. It is also soluble in dilute HCl acid and in chloroform. Xylazine is practically insoluble in water and in alkali solutions.
Biochem/physiol Actions
Xylazine when used along with ketamine is considered to be a potent and safe anaesthetic in experimental animal. It is known to elevate the hepatic release of glucose, which aggravates to hyperglycemia.
Mechanism of action
Xylazine is marketed as its hydrochloride salt as Rompun (100 mg/mL) and Anased (20 mg/mL) injectable solutions for intravenous administration to horses and dogs, respectively. The actions of xylazine may be reversed by the administration of yohimbine, an indolalkylamine alkaloid, that blocks those α2- adrenoreceptors that are stimulated by xylazine.
Side Effects
High doses of Xylazine can cause dizziness, vomiting, high blood sugar, blurry vision and even overdose deaths. If you inject xylazine into your body, you also may develop skin ulcers and severe wounds that can spread and worsen quickly.
Xylazine can lead to depression of the central nervous system along with other adverse effects. As for animals, they may show profound salivation following an injection with xylazine. Other adverse effects for dogs and cats include: muscle tremors, bradycardia with AV-block, hypotension, reduced respiratory rate, movement in response to strong auditory stimuli, and increased urination in cats.
Safety Profile
Poison by ingestion, subcutaneous, and intravenous routes. Human systemic effects: change in motor activity, fall in blood pressure, miosis, pleural thickening, pulse rate decrease, somnolence. When heated to decomposition it emits very toxic fumes of NOx and SOx.
Properties of Xylazine
| Melting point: | 140 C |
| Boiling point: | 334.2±52.0 °C(Predicted) |
| Density | 1.0905 (rough estimate) |
| refractive index | 1.5700 (estimate) |
| storage temp. | -20°C |
| solubility | Soluble in methanol at 50mg/ml. Also soluble in dilute acid solutions. |
| pka | 7.67±0.20(Predicted) |
| form | Powder |
| InChI | InChI=1S/C12H16N2S/c1-9-5-3-6-10(2)11(9)14-12-13-7-4-8-15-12/h3,5-6H,4,7-8H2,1-2H3,(H,13,14) |
| CAS DataBase Reference | 7361-61-7(CAS DataBase Reference) |
Safety information for Xylazine
| Signal word | Danger |
| Pictogram(s) |
 Skull and Crossbones Acute Toxicity GHS06 |
| GHS Hazard Statements |
H301:Acute toxicity,oral |
Computed Descriptors for Xylazine
| InChIKey | BPICBUSOMSTKRF-UHFFFAOYSA-N |
| SMILES | S1CCCN=C1NC1=C(C)C=CC=C1C |
Xylazine manufacturer
Noven Lifesciences Pvt Limited
Heer Pharma Pvt Ltd
New Products
(S)-3-Aminobutanenitrile hydrochloride 4-Methylphenylacetic acid N-Boc-D-alaninol N-BOC-D/L-ALANINOL Tert-butyl bis(2-chloroethyl)carbamate 3-Morpholino-1-(4-nitrophenyl)-5,6-dihydropyridin- 2(1H)-one Furan-2,5-Dicarboxylic Acid Tropic acid S-2-CHLORO PROPIONIC ACID ETHYL ISOCYANOACETATE 2-Bromo-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate 4-IODO BENZOIC ACID 3-NITRO-2-METHYL ANILINE 1-(2,4-DICHLOROPHENYL) ETHANAMINE (2-Hydroxyphenyl)acetonitrile 4-Bromopyrazole 5,6-Dimethoxyindanone 2-(Cyanocyclohexyl)acetic acid 4-methoxy-3,5-dinitropyridine 1-(4-(aminomethyl)benzyl)urea hydrochloride 2-aminopropyl benzoate hydrochloride diethyl 2-(2-((tertbutoxycarbonyl)amino) ethyl)malonate tert-butyl 4- (ureidomethyl)benzylcarbamate Ethyl-2-chloro((4-methoxyphenyl)hydrazono)acetateRelated products of tetrahydrofuran

You may like
-
 7361-61-7 Xylazine 98%View Details
7361-61-7 Xylazine 98%View Details
7361-61-7 -
 7361-61-7 Xylazine 99%View Details
7361-61-7 Xylazine 99%View Details
7361-61-7 -
 Xylazine 98% (GC) CAS 7361-61-7View Details
Xylazine 98% (GC) CAS 7361-61-7View Details
7361-61-7 -
 Xylazine CAS 7361-61-7View Details
Xylazine CAS 7361-61-7View Details
7361-61-7 -
 Xylazine CAS 7361-61-7View Details
Xylazine CAS 7361-61-7View Details
7361-61-7 -
 1975-50-4 98%View Details
1975-50-4 98%View Details
1975-50-4 -
 14714-50-2 (2-Hydroxyphenyl)acetonitrile 98+View Details
14714-50-2 (2-Hydroxyphenyl)acetonitrile 98+View Details
14714-50-2 -
 118753-70-1 98+View Details
118753-70-1 98+View Details
118753-70-1