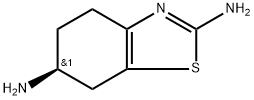
Pramipexole Impurity 4
Synonym(s):(S)-2-Amino-4,5,6,7-tetrahydro-6-(propylamino) benzothiazole dihydrochloride;(S)-2-amino-4,5,6,7-tetrahydro-6-(propylamino)benzothiazole dihydrochloride;PPX dihydrochloride;Pramipexole dihydrochloride monohydrate
- CAS NO.:104632-25-9
- Empirical Formula: C10H19Cl2N3S
- Molecular Weight: 284.25
- MDL number: MFCD00876894
- EINECS: 629-842-2
- SAFETY DATA SHEET (SDS)
- Update Date: 2025-10-28 16:00:23
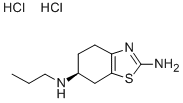
What is Pramipexole Impurity 4?
Description
Mirapex, an non ergot derivative, was launched in the US for treatment of Parkinson's disease. It can be prepared by two related routes, 5 steps and 3 steps, both involving an optical resolution with L-(+)-tartaric acid to afford the (S)-isomer. Mechanistically, it is a presynaptic dopamine D2 autoreceptor agonist which also activates D3-receptors. It is well absorbed from the GI and excreted in the urine essentially unchanged with a half-life of 8-12 hr. Mirapex had favorable effects on the symptoms without levodopa for patients with advanced PD and allowed a 27% reduction in use of levodopa in combination therapy. It had an adverse events profile similar to other dopamine agonists and in advanced patients reduced the mean "on-off" hr/day from 6 to 4. It was safe but not efficacious in all patients.
Description
(S)-Pramipexole is a dopamine D2S, D2L, D3, and D4 receptor agonist (EC50s = 426.58, 338.84, 2.24, and 128.82 nM, respectively, in a [35S]GTPγS binding assay). It is also a partial agonist of α2A-adrenergic receptors (α2A-ARs; EC50 = 3,548.13 nM). (S)-Pramipexole is selective for dopamine D2-4 receptors (Kis = 954.99, 1,698.24, 12.59, 128.82 nM for for D2S, D2L, D3, and D4 receptors, respectively, in a radioligand binding assay) over D1 and D5 receptors (Kis = >10,000 nM for both). It prevents MPTP-induced decreases in the number of dopaminergic neurons in the substantia nigra pars compacta in common marmosets when administered at a dose of 60 μg/kg per day before, during, and after administration of MPTP. Formulations containing (S)-pramipexole have been used in the treatment of Parkinson''s disease and restless legs syndrome.
Chemical properties
White Crystalline Powder
Originator
Boehringer Ingelheim (Germany)
The Uses of Pramipexole Impurity 4
A dopamine-D2-receptor agonist. Antiparkinsonian
The Uses of Pramipexole Impurity 4
A dopamine-D2-receptor agonist. Antiparkinsonian.
What are the applications of Application
(S)-Pramipexole Dihydrochloride is a D2DR and D3DR agonist
Definition
ChEBI: A hydrochloride that is the anhydrous dihydrochloride salt of pramipexole.
Manufacturing Process
75.5 g (0.5 mol) of 4-aminocyclohexanol hydrochloride and 74.0 g (0.5 mol) of phthalic acid anhydride are mixed with 65 g (0.5 mol) of ethyldiisopropyl amine and 1000 ml of toluene and boiled for 36 hours with a water separator. Then water is added, the toluene phase is separated off and the aqueous phase is extracted several times with chloroform. The organic phases are combined, dried and concentrated. The concentrated residue is recrystallised from isopropanol and 4-(phthalimido)-cyclohexanol was obtained. Yield: 95 g (77.8%). Melting point 175°-176°C.
95 g (0.388 mol) of 4-(phthalimido)-cyclohexanol are dissolved in 600 ml of chloroform and, after the addition of 450 ml of water and 120 ml of sulfuric acid, 90 g (0.3 mol) of potassium dichromate are added in batches. The internal temperature of the mixture is maintained at between 25° and 30°C by slight cooling. The mixture is stirred for a further 3 hours, then the chloroform phase is separated off and the mixture extracted twice more with chloroform. After drying and concentration of the extracts 82 g (86.9%) of 4(phthalimido)-cyclohexanone was obtained.
48.6 g (0.2 mol) of 4-(phthalimido)cyclohexanone are dissolved in glacial acetic acid, mixed with 36% of hydrobromic acid in glacial acetic acid and then 32 g (0.2 mol) of bromine in glacial acetic acid is added dropwise with cooling. The mixture is then concentrated by evaporation in vacuo and the residue is triturated several times with diethylether. The ether extracts are discarded and the residue is dissolved in of ethanol. After thiourea have been added the mixture is refluxed for 5 hours. It is then concentrated by evaporation, made alkaline with sodium hydroxide solution and extracted with chloroform. After drying and concentration of the extracts, the residue is purified by column chromatography on silica gel (eluant: chloroform/methanol = 1/1). The 2-amino-6-phthalimido-4,5,6,7-tetrahydro-benzthiazol was obtained. Melting point 244-246°C, dec. Yield: 30 g (50%).
9.5 g (31.7 mmol) of 2-amino-6-phthalimido-4,5,6,7-tetrahydro-benzthiazole are suspended in 100 ml of ethanol and, after the addition of 1.8 g (36 mmol) of hydrazine hydrate, refluxed for 2 hours. The mixture is then concentrated and purified by column chromatography on silica gel using methanol as eluant. The 2,6-diamino- 4,5,6,7-tetrahydro-benzthiazole was obtained.
To a solution of 2,6-diamino- 4,5,6,7-tetrahydro-benzthiazole in dimethylformamide are added n-propanal and the mixture is heated to 50°C for 1 hour. After cooling, the reaction solution is mixed with sodium borohydride and heated to 50°C for 30 min. The solvent is largely eliminated in vacuo. Whilst cooling with ice, the residue is mixed with water and 2 N hydrochloric acid until a pH of 1 is obtained. The aqueous solution is exwith ethylacetate and the organic phase discarded. The aqueous phase is mixed with potassium carbonate until an alkaline reaction is obtained and then extracted with ethyl acetate. The organic phase is dried and concentrated. The 2-amino-6-n-propylamino-4,5,6,7-tetrahydro-benzthiazole dihydrochloride crystallizes out when ethereal hydrochloric acid is added. Yield: 42%. Melting point: 286°-288°C.
brand name
Mirapex (Boehringer Ingelheim).
Therapeutic Function
Antiparkinsonian, Antipsychotic
General Description
Pramipexole dihydrochloride,(S)-2-amino-6-propylamino-dihydrochloride(Mirapex), is a white to off-white powder soluble in water,slightly soluble in methanol and ethanol, and practically insolublein dichloromethane. Following oral administration,pramipexole is readily absorbed. Pharmacokinetic propertiesdiffer between men and women, with area under the curve(AUC) for each dose level being 35% to 43% greater inwomen, mainly because of a 24% to 27% lower oral clearance.The drug undergoes minimal hepatic biotransformationand is excreted virtually unchanged in the urine by the renaltubular secretion. Pramipexole interacts with drugs excretedby renal tubular secretion (H2-antagonists, diuretics, verapamil,quinidine, quinine), which leads to a decreased clearanceof pramipexole.44,45 Pramipexole is indicated fortreatment of the signs and symptoms of idiopathic PD, aloneor in combination with levodopa. It is also indicated for symptomatictreatment of moderate to severe idiopathic restlesslegs syndrome (RLS).
Biochem/physiol Actions
Pramipexole is a dopamine agonist active at D3 and D2 receptor subtypes. Pramipexole has been found to have neuroprotective effects independent of its dopamine receptor agonism. It reduces mitochondrial reactive oxygen species (ROS) production and inhibits the activation of apoptotic pathways.
Storage
room temperature (desiccate)
Properties of Pramipexole Impurity 4
| Melting point: | 288-290°C |
| storage temp. | 2-8°C |
| solubility | H2O: >20mg/mL |
| form | powder |
| color | white to off-white |
| CAS DataBase Reference | 104632-25-9(CAS DataBase Reference) |
Safety information for Pramipexole Impurity 4
| Signal word | Warning |
| Pictogram(s) |
 Exclamation Mark Irritant GHS07 |
| GHS Hazard Statements |
H302:Acute toxicity,oral H336:Specific target organ toxicity,single exposure; Narcotic effects |
| Precautionary Statement Codes |
P261:Avoid breathing dust/fume/gas/mist/vapours/spray. P264:Wash hands thoroughly after handling. P264:Wash skin thouroughly after handling. P270:Do not eat, drink or smoke when using this product. P271:Use only outdoors or in a well-ventilated area. P301+P312:IF SWALLOWED: call a POISON CENTER or doctor/physician IF you feel unwell. |
Computed Descriptors for Pramipexole Impurity 4
| InChIKey | QMNWXHSYPXQFSK-KLXURFKVSA-N |
Pramipexole Impurity 4 manufacturer
New Products
Indole Methyl Resin tert-butyl 9-methoxy-3-azaspiro[5.5]undecane-3-carboxylate Boc-His(Boc)-OH 2-CTC Resin 4-Chloro-7-tosy1-7Hpyrrolo[2,3-d]pyrimidine 5,7-Dibromo-1H-indole 2,5-dichloro-N-hydroxy-4,6-dimethylpyridine-3-carboximidamide 2,2-Dimethoxy-7-azaspiro[3.5]nonane hydrochloride 4-chloromethyl-5-methyl-1,3-dioxol-2-one (DMDO-Cl) R-2-BENZYLOXY PROPIONIC ACID 1,1’-CARBONYLDIIMIDAZOLE 1,1’-CARBONYLDI (1,2-4 TRIAZOLE) N-METHYL INDAZOLE-3-CARBOXYLIC ACID 4-((2-hydroxyethyl)thio)benzoic acid 1-(TERT-BUTOXYCARBONYL)-2-PYRROLIDINONE Methyl 6-methylnicotinate 3-Pyridineacrylic acid tert-Butyl carbazate TETRAHYDRO-2H-PYRAN-3-OL 2-((4-morpholinophenylamino) (methylthio) methylene) malononitrile 3-(4-morpholinophenylamino)-5-amino-1H-pyrazole-4-carbonitrile 2,4-dihydroxybenzaldehyde 1,3-Diethyl-1,3-Diphenylurea Methyl 2-methylquinoline-6-carboxylateRelated products of tetrahydrofuran


You may like
-
 104632-25-9 98%View Details
104632-25-9 98%View Details
104632-25-9 -
 PRAMIPEXOLE DI HCL 99%View Details
PRAMIPEXOLE DI HCL 99%View Details -
 104632-25-9 Pramipexole dihydrochloride 98%View Details
104632-25-9 Pramipexole dihydrochloride 98%View Details
104632-25-9 -
 104632-25-9 98%View Details
104632-25-9 98%View Details
104632-25-9 -
 Pramipexole dihydrochloride 99%View Details
Pramipexole dihydrochloride 99%View Details
104632-25-9 -
 104632-25-9 Pramipexole dihydrochloride 98%View Details
104632-25-9 Pramipexole dihydrochloride 98%View Details
104632-25-9 -
 Pramipexole dihydrochloride 98.00% CAS 104632-25-9View Details
Pramipexole dihydrochloride 98.00% CAS 104632-25-9View Details
104632-25-9 -
 Pramipexole dihydrochloride CAS 104632-25-9View Details
Pramipexole dihydrochloride CAS 104632-25-9View Details
104632-25-9
