Propafenone Hydrochloride
Synonym(s):1-[2-(2-Hydroxy-3-(propylamino)propoxy)phenyl]-3-phenyl-1-propanone hydrochloride
- CAS NO.:34183-22-7
- Empirical Formula: C21H28ClNO3
- Molecular Weight: 377.9
- MDL number: MFCD00079243
- EINECS: 251-867-9
- SAFETY DATA SHEET (SDS)
- Update Date: 2025-09-09 10:27:52
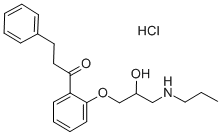
What is Propafenone Hydrochloride?
Description
Propafenone hydrochloride is a class I anti-arrhythmic agent with basic local anaesthetic and membrane-stabilizing properties. Some P-adrenergic blocking action has also been described. Propafenone decreases the depolarization velocity and slows conduction in the His-Purkinje system with resultant increase in the PR interval and the QRS complex. Propafenone is used in the treatment of paroxysmal supraventricular tachycardias and ventricular arrhythmias.
Propafenone should not be used in uncontrolled cardiac failure, severe obstructive pulmonary disease or marked hypotension. Propafenone may worsen myasthenia gravis.
Chemical properties
White Solid
The Uses of Propafenone Hydrochloride
Propafenone hydrochloride is a class IC antiarrhythmic agent used for the management of severe ventricular and supraventricular arrhythmias. It has beta-blocking and weak calcium channel blocking properties, as well as some negative inotropic activity. Propafenone is in a class of medications called antiarrhythmics. It works by acting on the heart muscle to improve the heart's rhythm.
The Uses of Propafenone Hydrochloride
Sodium channel blocker. Antiarrhythmic (class IC)
What are the applications of Application
Propafenone Hydrochloride is a sodium channel protein inhibitor
Definition
ChEBI: Propafenone hydrochloride is a hydrochloride that is the monohydrochloride salt of propafenone. It is a class 1C antiarrhythmic drug with local anesthetic effects, and is used in the management of supraventricular and ventricular arrhythmias. It has a role as an anti-arrhythmia drug. It contains a propafenone(1+).
brand name
Rythmol (Reliant).
Pharmacokinetics
Propafenone hydrochloride (Rythmol(R)) is similar in action to flecainide. It reduces the fast inward sodium current in Purkinje fibres and to a lesser extent in myocardial fibres. Unlike other class l drugs, propafenone has mild B-blocking effects. This may contribute to its overall effects on the conduction system. lt is also believed to have calcium channel-blocking effects, which may contribute to its mild negative inotropic effects.
Clinical Use
Anti-arrhythmic agent:
Ventricular arrhythmias
Paroxysmal supraventricular tachyarrhythmias,
(including paroxysmal atrial flutter or fibrillation,
and paroxysmal re-entrant tachycardias involving
the AV node or accessory pathway) where standard
therapy has failed or is unsuitable
Drug interactions
Potentially hazardous interactions with other drugs
Anti-arrhythmics: increased myocardial depression
with other anti-arrhythmics.
Antibacterials: increased metabolism with rifampicin
(reduced effect).
Anticoagulants: enhanced anticoagulant effect of
coumarins.
Antidepressants: increased risk of arrhythmias
with tricyclics; metabolism of propafenone possibly
inhibited by paroxetine (increased risk of toxicity).
Antihistamines: increased risk of ventricular
arrhythmias with mizolastine - avoid.
Antipsychotics: increased risk of ventricular
arrhythmias with antipsychotics that prolong the QT
interval.
Antivirals: concentration of propafenone increased
by saquinavir and ritonavir and possibly by
fosamprenavir, increased risk of ventricular
arrhythmias - avoid; use with caution with
telaprevir.
Beta-blockers: increased myocardial depression;
increased concentration of metoprolol and
propranolol.
Cardiac glycosides: increased digoxin concentration -
halve digoxin dose.
Ciclosporin: possibly increased ciclosporin
concentration.
Ulcer-healing drugs: levels increased by cimetidine.
Metabolism
Propafenone is hepatically metabolised mainly by
CYP2D6 isoenzyme but also to a small extent by
CYP1A2 and CYP3A4. This forms 2 active metabolites,
5-hydroxypropafenone and N-depropylpropafenone and
some inactive ones. Propafenone and its metabolites also
undergo glucuronidation. The extent of metabolism is
genetically determined.
Propafenone is excreted in the urine and faeces mainly in
the form of conjugated metabolites.
Properties of Propafenone Hydrochloride
| Melting point: | 165-1670C |
| storage temp. | 2-8°C |
| solubility | Slightly soluble in cold water, soluble in methanol and in hot water, practically insoluble in ethanol (96 per cent). |
| form | neat |
| form | Solid |
| color | White to Off-White |
| λmax | 303nm(MeOH)(lit.) |
| Merck | 14,7794 |
| CAS DataBase Reference | 34183-22-7(CAS DataBase Reference) |
Safety information for Propafenone Hydrochloride
| Signal word | Warning |
| Pictogram(s) |
 Exclamation Mark Irritant GHS07 |
| GHS Hazard Statements |
H302:Acute toxicity,oral |
Computed Descriptors for Propafenone Hydrochloride
Propafenone Hydrochloride manufacturer
New Products
Indole Methyl Resin tert-butyl 9-methoxy-3-azaspiro[5.5]undecane-3-carboxylate Boc-His(Boc)-OH 2-CTC Resin 4-Chloro-7-tosy1-7Hpyrrolo[2,3-d]pyrimidine 5,7-Dibromo-1H-indole 2,5-dichloro-N-hydroxy-4,6-dimethylpyridine-3-carboximidamide 2,2-Dimethoxy-7-azaspiro[3.5]nonane hydrochloride 4-chloromethyl-5-methyl-1,3-dioxol-2-one (DMDO-Cl) R-2-BENZYLOXY PROPIONIC ACID 1,1’-CARBONYLDIIMIDAZOLE 1,1’-CARBONYLDI (1,2-4 TRIAZOLE) N-METHYL INDAZOLE-3-CARBOXYLIC ACID 4-((2-hydroxyethyl)thio)benzoic acid 1-(TERT-BUTOXYCARBONYL)-2-PYRROLIDINONE Methyl 6-methylnicotinate 3-Pyridineacrylic acid tert-Butyl carbazate TETRAHYDRO-2H-PYRAN-3-OL 2-((4-morpholinophenylamino) (methylthio) methylene) malononitrile 3-(4-morpholinophenylamino)-5-amino-1H-pyrazole-4-carbonitrile 2,4-dihydroxybenzaldehyde 1,3-Diethyl-1,3-Diphenylurea Methyl 2-methylquinoline-6-carboxylateRelated products of tetrahydrofuran


You may like
-
 34183-22-7 98%View Details
34183-22-7 98%View Details
34183-22-7 -
 Propafenone Hydrochloride CAS 34183-22-7View Details
Propafenone Hydrochloride CAS 34183-22-7View Details
34183-22-7 -
 Propafenone CAS 34183-22-7View Details
Propafenone CAS 34183-22-7View Details
34183-22-7 -
 Propafenone hydrochloride solution CAS 34183-22-7View Details
Propafenone hydrochloride solution CAS 34183-22-7View Details
34183-22-7 -
 Propafenone hydrochloride CAS 34183-22-7View Details
Propafenone hydrochloride CAS 34183-22-7View Details
34183-22-7 -
 Propafenone hydrochloride CAS 34183-22-7View Details
Propafenone hydrochloride CAS 34183-22-7View Details
34183-22-7 -
 Propafenone Hydrochloride 34183 22 7View Details
Propafenone Hydrochloride 34183 22 7View Details
34183-22-7 -
 Propafenone HydrochlorideView Details
Propafenone HydrochlorideView Details
34183-22-7
