Potassium phthalimide
Synonym(s):1,3-Dihydro-1,3-dioxoisoindole potassium salt;Potassium phthalimide;PPI
- CAS NO.:1074-82-4
- Empirical Formula: C8H4KNO2
- Molecular Weight: 185.22
- MDL number: MFCD00005887
- EINECS: 214-046-6
- SAFETY DATA SHEET (SDS)
- Update Date: 2025-01-27 09:38:02
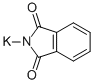
What is Potassium phthalimide?
Chemical properties
slight yellow-green to white powder
The Uses of Potassium phthalimide
Condensation of phthalimide potassium with organic halide in dimethylformamide has been reported. Reaction of potassium phthalimide and sulfur monochloride in petroleum ether has been studied.
Description
Potassium phthalimide is a versatile intermediate in organic synthesis with a range of applications. It is utilized in the synthesis of N-alkylated phthalimides, which are key in the preparation of primary amines through the Gabriel synthesis method and subsequent hydrolysis reaction. Beyond its role in amine synthesis, potassium phthalimide is also an essential intermediate in the production of synthetic indigo, pigments, dyes, and pharmaceuticals. Furthermore, it functions as an organocatalyst, facilitating the cyanosilylation of various carbonyl compounds under mild conditions. Additionally, it is employed as a reagent for converting allyl- and alkyl halides into protected primary amines, highlighting its importance in the synthesis of a variety of chemical compounds.
What are the applications of Application
Potassium phthalimide is used as an intermediate in the synthesis of N-alkylated phthalimides, which is involved in the preparation of primary amines (Gabriel synthesis) by the hydrolysis reaction. It is also used as an intermediate for synthetic indigo, pigments, dyes and pharmaceuticals. Further, it is employed as an organocatalyst for the cyanosilylation of various carbonyl compounds under extremely mild conditions. In addition to this, it serves as a reagent for the transformation of allyl- and alkyl halides into protected primary amines.
Purification Methods
The solid may contain phthalimide and K2CO3 from hydrolysis. If too much hydrolysis has occurred (this can be checked by extraction with cold Me2CO in which the salt is insoluble, evaporate the Me2CO and weigh the residue), it would be better to prepare it afresh. If little hydrolysis has occurred, then recrystallise it from a large volume of EtOH, and wash the solid with a little Me2CO and dry it in a continuous vacuum to constant weight. [Salzerg & Supriawski Org Synth Coll Vol I 119 1941, Raman & IR: Hase J Mol Struct 48 33 1978, Dykman Chem Ind (London) 40 1972, IR, NMR: Assef et al. Bull Soc Chim Fr II 167 1979, Beilstein 21/10 V 270.]
Properties of Potassium phthalimide
| Melting point: | >300°C |
| Boiling point: | 366C |
| Density | 1.63 |
| storage temp. | Inert atmosphere,Room Temperature |
| solubility | water: soluble50mg/mL, clear to slightly hazy, colorless to yellow |
| form | Crystalline Powder |
| color | White to yellow or greenish |
| PH | pH (50g/l, 25℃) : 10.5~12.5 |
| Water Solubility | Soluble in water. |
| Sensitive | Moisture Sensitive |
| BRN | 3598719 |
| CAS DataBase Reference | 1074-82-4(CAS DataBase Reference) |
| EPA Substance Registry System | Potassium phthalimide (1074-82-4) |
Safety information for Potassium phthalimide
| Signal word | Warning |
| Pictogram(s) |
 Exclamation Mark Irritant GHS07 |
| GHS Hazard Statements |
H315:Skin corrosion/irritation H319:Serious eye damage/eye irritation |
| Precautionary Statement Codes |
P264:Wash hands thoroughly after handling. P264:Wash skin thouroughly after handling. P280:Wear protective gloves/protective clothing/eye protection/face protection. P302+P352:IF ON SKIN: wash with plenty of soap and water. P305+P351+P338:IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. Continuerinsing. P332+P313:IF SKIN irritation occurs: Get medical advice/attention. P337+P313:IF eye irritation persists: Get medical advice/attention. |
Computed Descriptors for Potassium phthalimide
Potassium phthalimide manufacturer
ARRAKIS INDUSTRIES LLP
ASM Organics
New Products
Methyl (R)-1-Boc-4,4-difluoropyrrolidine-2-carboxylate 2,2-Difluoropropylamine hydrochloride tert-butyl 3-bromoazetidine-1-carboxylate (R)-1-Boc-3-hydroxypyrrolidine DIFLUOROACETIC ANHYDRIDE 2,2-Difluoropropionic acid Diallylamine, 99% Calcium hydroxide, 95% Aluminum oxide, basic 2-Bromophenylacetonitrile, 97% L-tert-Leucine,97% N-Hydroxy-2-methylpropanimidamide 4-(3,4-Dichlorophenyl)-3,4-Dihydro-N-Methyl-1-(2H)-Naphthalenimine (Schiff Base) 2-AMINO-3,5-DIBROMO BENZALDEHYDE [ADBA] L-Glutamic Acid Dimethyl Ester Hcl 10-Methoxy-5H-dibenz[b,f]azepine 5-Cyanophthalide N, N-Carbonyldiimidazole (CDI) Dibenzoyl Peroxide Titanium Dioxide 2-(Methylthio) Benzonitrile Sodium Acetate Anhydrous Allopurinol 1,5-DibromopentaneRelated products of tetrahydrofuran
You may like
-
 1074-82-4 Potassium phthalimide 98%View Details
1074-82-4 Potassium phthalimide 98%View Details
1074-82-4 -
 Potassium phthalimide 98%View Details
Potassium phthalimide 98%View Details
1074-82-4 -
 Potassium phthalimide CAS 1074-82-4View Details
Potassium phthalimide CAS 1074-82-4View Details
1074-82-4 -
 Potassium phthalimide CAS 1074-82-4View Details
Potassium phthalimide CAS 1074-82-4View Details
1074-82-4 -
 Potassium phthalimide, pract CAS 1074-82-4View Details
Potassium phthalimide, pract CAS 1074-82-4View Details
1074-82-4 -
 Phthalimide Potassium Salt CAS 1074-82-4View Details
Phthalimide Potassium Salt CAS 1074-82-4View Details
1074-82-4 -
 Potassium phthalimide 95% CAS 1074-82-4View Details
Potassium phthalimide 95% CAS 1074-82-4View Details
1074-82-4 -
 POTASSIUM PHTHALIMIDE CAS 1074-82-4View Details
POTASSIUM PHTHALIMIDE CAS 1074-82-4View Details
1074-82-4
