1074-82-4
Product Name:
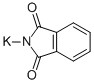
Potassium phthalimide
Formula:
C8H4KNO2
Synonyms:
1,3-Dihydro-1,3-dioxoisoindole potassium salt;Potassium phthalimide;PPI
Inquiry
CHEMICAL AND PHYSICAL PROPERTIES
| Physical Description | White powder; [Alfa Aesar MSDS] |
|---|---|
| Chemical Classes | Nitrogen Compounds -> Other Nitrogen Rings |
SAFETY INFORMATION
| Signal word | Warning |
|---|---|
| Pictogram(s) |
 Exclamation Mark Irritant GHS07 |
| GHS Hazard Statements |
H315:Skin corrosion/irritation H319:Serious eye damage/eye irritation |
| Precautionary Statement Codes |
P264:Wash hands thoroughly after handling. P264:Wash skin thouroughly after handling. P280:Wear protective gloves/protective clothing/eye protection/face protection. P302+P352:IF ON SKIN: wash with plenty of soap and water. P305+P351+P338:IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. Continuerinsing. P332+P313:IF SKIN irritation occurs: Get medical advice/attention. P337+P313:IF eye irritation persists: Get medical advice/attention. |
COMPUTED DESCRIPTORS
| Molecular Weight | 185.22 g/mol |
|---|---|
| Hydrogen Bond Donor Count | 0 |
| Hydrogen Bond Acceptor Count | 3 |
| Rotatable Bond Count | 0 |
| Exact Mass | 184.98790986 g/mol |
| Monoisotopic Mass | 184.98790986 g/mol |
| Topological Polar Surface Area | 35.1 Ų |
| Heavy Atom Count | 12 |
| Formal Charge | 0 |
| Complexity | 195 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 0 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently-Bonded Unit Count | 2 |
| Compound Is Canonicalized | Yes |
PRODUCT INTRODUCTION
Description
Potassium phthalimide is used as an intermediate in the synthesis of N-alkylated phthalimides, which is involved in the preparation of primary amines (Gabriel synthesis) by the hydrolysis reaction. It is also used as an intermediate for synthetic indigo, pigments, dyes and pharmaceuticals. Further, it is employed as an organocatalyst for the cyanosilylation of various carbonyl compounds under extremely mild conditions. In addition to this, it serves as a reagent for the transformation of allyl- and alkyl halides into protected primary amines.
