Potassium Phosphate Dibasic
Synonym(s):Dipotassium phosphate;Potassium phosphate dibasic;Dipotassium hydrogenphosphate;sec.-Potassium phosphate;Potassium Hydrogen Phosphate, Dipotassium Phosphate
- CAS NO.:7758-11-4
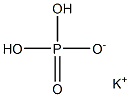
- Empirical Formula: K2HPO4
- Molecular Weight: 174.18
- MDL number: MFCD00011383
- EINECS: 231-834-5
- SAFETY DATA SHEET (SDS)
- Update Date: 2025-12-17 09:49:53
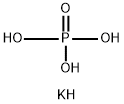
What is Potassium Phosphate Dibasic?
Absorption
Potassium salts are well absorbed from gastro intestinal tract. Net phosphorus absorption may occur in the small intestine in some species but is primarily a function of the colon in horses.
Toxicity
LD50 not available. Toxic effects in humans: Irritated skin, eye. Gastro intestinal irritation with nausea, vomiting, diarrhea, abdominal discomfort
Description
Potassium Phosphate Dibasic is a common source of phosphorus and potassium, which is often used as a fertilizer. It is also widely applied in the food industry, such as food additive and electrolyte replenisher for workout supplement. Another usage of Potassium Phosphate Dibasic is as a medicine, who serves as a diuretic or laxative. Besides, it is employed in the production of imitation dairy creamers to prevent coagulation and used in certain powders to prepare beverages. In addition, it is commonly seen in chemical laboratories for producing buffer solutions and trypticase soy agar which is used to make agar plates for culturing bacteria.
Physical properties
White amorphous powder; deliquesces; decomposes on heating; converts to pyrophosphate when ignited; very soluble in water, 167 g/100mL at 20°C; very soluble in alcohol; aqueous solution slightly alkaline.
The Uses of Potassium Phosphate Dibasic
Potassium Phosphate Dibasic is the dipotassium salt of phosphoric acid which functions as a stabilizing salt, buffer, and sequestrant. It is mildly alkaline with a ph of 9 and is soluble in water with a solubility of 170 g/100 ml of water at 25°c. It improves the colloidal solubility of proteins. It acts as a buffer against variation in ph. For example, it is used in coffee whiteners as a buffer against ph variation in hot coffee and to prevent feathering. It also functions as an emulsifier in specified cheeses and as a buffering agent for processed foods. It is also termed dipotassium monohydrogen orthophosphate and dipotassium monophosphate.
What are the applications of Application
Dipotassium hydrogen phosphate is widely used in molecular biology, biochemistry, and chromatography. Potassium phosphate dibasic solution is a convenient 1M stock solution for making phosphate buffers. It is also used as a buffering agent in antifreeze solutions; nutrient in the culturing of antibiotics; ingredient of instant fertilizers; as sequestrant in the preparation of non-dairy powdered coffee creams.
Background
Dipotassium phosphate (K2HPO4) is a highly water-soluble salt often used as a fertilizer and food additive as a source of phosphorus and potassium as well as a buffering agent.
Indications
Dipotassium phosphate is used in imitation dairy creamers, dry powder beverages, mineral supplements, and starter cultures as an additive. It is used in non-dairy creamers to prevent coagulation. Dipotassium phosphate is also used to make buffer solutions and it is used in the production of trypticase soy agar which is used to make agar plates for culturing bacteria.
Definition
ChEBI: Dipotassium hydrogen phosphate is a potassium salt that is the dipotassium salt of phosphoric acid. It has a role as a buffer. It is a potassium salt and an inorganic phosphate.
Preparation
Dipotassium phosphate is prepared by partial neutralization of phosphoric acid with potassium hydroxide, followed by crystallization:
H3PO4+ 2KOH →K2HPO4+ 2H2O.
What are the applications of Application
Potassium Phosphate Dibasic can be used as corrosion inhibitor of antifreeze, nutrient of antibiotic culture medium, phosphorus and potassium regulator of fermentation industry, feed additive, medicine, fermentation, bacterial culture and preparation of potassium pyrophosphate, as feed phosphorus supplement additive. Potassium hydrogen phosphate can also be used as water treatment agent, microorganism, fungus culture agent and other purposes. It is often used as analytical reagent and buffer. It is also used in pharmaceutical industry. In food industry, it is used as raw material for preparing alkaline water for pasta products, fermentation agent, flavoring agent, bulking agent, mild alkaline agent for dairy products and yeast food. Potassium Phosphate Dibasic can be used as buffer, chelating agent and analytical reagent. Buffers and pharmaceuticals. Potassium Phosphate Dibasic can be used for boiler water treatment. In medicine and fermentation industry, Potassium Phosphate Dibasic can be used as phosphorus and potassium regulator and bacterial culture medium. It is the raw material for producing potassium pyrophosphate. It can be used as liquid fertilizer and corrosion inhibitor of ethylene glycol antifreeze. Feed grade is used as feed nutritional supplement. Potassium dihydrogen phosphate can be used as a product quality improver, which can improve the complex metal ions, pH value and ionic strength of food, so as to improve the bonding force and water holding capacity of food. China stipulates that Potassium Phosphate Dibasic can be used for fat planting powder, with a maximum dosage of 19.9g/kg.
General Description
Potassium phosphate dibasic is a useful biological buffer having a pH in the range of 8.5-9.6. Potassium phosphate is present in various forms: monobasic (KH2PO4), dibasic (KH2PO4), and tribasic (K3PO4). Potassium phosphate buffers are widely employed in equilibrium dialysis, high performance liquid chromatography (HPLC) and affinity capillary electrophoresis studies.
Pharmacokinetics
Phosphate is a major intracellular anion which participates in providing energy for metabolism of substances and contributes to important metabolic and enzymatic reactions in almost all organs and tissues. Phosphate exerts a modifying influence on calcium concentrations, a buffering effect on acid-base equilibrium, and has a major role in the renal excretion of hydrogen ions.
Metabolism
Phosphate is a major intracellular anion which participates in providing energy for metabolism of substances and contributes to important metabolic and enzymatic reactions in almost all organs and tissues.
References
https://en.wikipedia.org/wiki/Dipotassium_phosphate
https://pubchem.ncbi.nlm.nih.gov/compound/Dipotassium_hydrogen_phosphate#section=Other-Identifiers
https://www.isitbadforyou.com/questions/is-dipotassium-phosphate-bad-for-you
Properties of Potassium Phosphate Dibasic
| Melting point: | 340 °C |
| Density | 2,44 g/cm3 |
| storage temp. | Store at +5°C to +30°C. |
| solubility | H2O: 1 M at 20 °C, clear, colorless |
| form | Solid |
| appearance | White powder |
| color | White |
| Odor | Odorless |
| PH | 8.5-9.6 (25℃, 50mg/mL in H2O) |
| pka | (1) 2.15, (2) 6.82, (3) 12.38 (at 25℃) |
| PH Range | 8.8 |
| Water Solubility | 1600 g/L (20 ºC) |
| Sensitive | Hygroscopic |
| λmax | λ: 260 nm Amax: ≤0.20 λ: 280 nm Amax: ≤0.15 |
| Decomposition | >465°C |
| Merck | 14,7658 |
| Stability: | Stable. Hygroscopic. |
| CAS DataBase Reference | 7758-11-4(CAS DataBase Reference) |
| EPA Substance Registry System | Dipotassium phosphate (7758-11-4) |
Safety information for Potassium Phosphate Dibasic
| Pictogram(s) |
 Exclamation Mark Irritant GHS07 |
| GHS Hazard Statements |
H319:Serious eye damage/eye irritation |
| Precautionary Statement Codes |
P264:Wash hands thoroughly after handling. P264:Wash skin thouroughly after handling. P305+P351+P338:IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. Continuerinsing. P337+P313:IF eye irritation persists: Get medical advice/attention. |
Computed Descriptors for Potassium Phosphate Dibasic
| InChIKey | ZPWVASYFFYYZEW-UHFFFAOYSA-L |
Potassium Phosphate Dibasic manufacturer
JSK Chemicals
H. K. Group
New Products
Indole Methyl Resin tert-butyl 9-methoxy-3-azaspiro[5.5]undecane-3-carboxylate Boc-His(Boc)-OH 2-CTC Resin 4-Chloro-7-tosy1-7Hpyrrolo[2,3-d]pyrimidine 5,7-Dibromo-1H-indole 2,5-dichloro-N-hydroxy-4,6-dimethylpyridine-3-carboximidamide 2,2-Dimethoxy-7-azaspiro[3.5]nonane hydrochloride 4-chloromethyl-5-methyl-1,3-dioxol-2-one (DMDO-Cl) R-2-BENZYLOXY PROPIONIC ACID 1,1’-CARBONYLDIIMIDAZOLE 1,1’-CARBONYLDI (1,2-4 TRIAZOLE) N-METHYL INDAZOLE-3-CARBOXYLIC ACID 4-((2-hydroxyethyl)thio)benzoic acid 1-(TERT-BUTOXYCARBONYL)-2-PYRROLIDINONE Methyl 6-methylnicotinate 3-Pyridineacrylic acid tert-Butyl carbazate TETRAHYDRO-2H-PYRAN-3-OL 2-((4-morpholinophenylamino) (methylthio) methylene) malononitrile 3-(4-morpholinophenylamino)-5-amino-1H-pyrazole-4-carbonitrile 2,4-dihydroxybenzaldehyde 1,3-Diethyl-1,3-Diphenylurea Methyl 2-methylquinoline-6-carboxylateRelated products of tetrahydrofuran
You may like
-
 Potassium Phosphate Dibasic 99%View Details
Potassium Phosphate Dibasic 99%View Details -
 Potassium phosphate, dibasic 99%View Details
Potassium phosphate, dibasic 99%View Details -
 Potassium phosphate, dibasic 98%View Details
Potassium phosphate, dibasic 98%View Details -
 Potassium hydrogen phosphate CAS 7758-11-4View Details
Potassium hydrogen phosphate CAS 7758-11-4View Details
7758-11-4 -
 Potassium hydrogen phosphate CAS 7758-11-4View Details
Potassium hydrogen phosphate CAS 7758-11-4View Details
7758-11-4 -
 DI-POTASSIUM HYDROGEN ORTHOPHOSPHATE ANHYDROUS 2139900 99%View Details
DI-POTASSIUM HYDROGEN ORTHOPHOSPHATE ANHYDROUS 2139900 99%View Details
2139900 -
 POTASSIUM PHOSPHATE DIBASIC ANHYDROUS Extra Pure CASView Details
POTASSIUM PHOSPHATE DIBASIC ANHYDROUS Extra Pure CASView Details -
 POTASSIUM PHOSPHATE DIBASIC ANHYDROUS Molecular Biology CASView Details
POTASSIUM PHOSPHATE DIBASIC ANHYDROUS Molecular Biology CASView Details
