Potassium oleate
Synonym(s):Oleic acid potassium salt
- CAS NO.:143-18-0
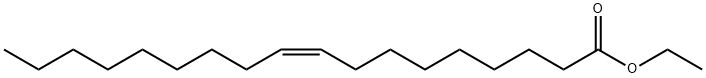
- Empirical Formula: C18H33KO2
- Molecular Weight: 320.55
- MDL number: MFCD00064243
- EINECS: 205-590-5
- SAFETY DATA SHEET (SDS)
- Update Date: 2025-01-27 09:38:02
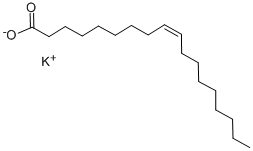
What is Potassium oleate?
Chemical properties
Gray-tan paste. Soluble in water and alcohol. Combustible.
The Uses of Potassium oleate
Potassium Oleate is the potassium salt of oleic acid. it is used as a binder, emulsifier, and anticaking agent.
The Uses of Potassium oleate
Potassium Oleate can be used as a compound disinfectant.
What are the applications of Application
Potassium oleate is a synthesis reagent
General Description
Brown solid or clear to amber liquid with a soapy odor. Sinks and mixes slowly with water.
Air & Water Reactions
Water soluble. Gives basic aqueous solution.
Reactivity Profile
Salts, basic, such as OLEIC ACID, [POTASSIUM SALT], are generally soluble in water. The resulting solutions contain moderate concentrations of hydroxide ions and have pH's greater than 7.0. They react as bases to neutralize acids. These neutralizations generate heat, but less or far less than is generated by neutralization of the bases in reactivity group 10 (Bases) and the neutralization of amines. They usually do not react as either oxidizing agents or reducing agents but such behavior is not impossible.
Health Hazard
Inhalation of dust causes irritation of nose and throat, coughing, and sneezing. Ingestion causes mild irritation of mouth and stomach. Contact with eyes causes irritation.
Fire Hazard
Special Hazards of Combustion Products: Irritating vapors and toxic gases, such as carbon dioxide and carbon monoxide, may be formed when involved in fire.
Safety Profile
An eye irritant. When heated to decomposition it emits toxic fumes of K2O.
Purification Methods
Recrystallise it from EtOH (1g/mL). [Beilstein 2 H 465, 2 I 196, 2 I 202, 2 II 436, 2 III 1404, 2 IV 1646.]
Properties of Potassium oleate
| Density | >1.1 g/cm3 |
| Flash point: | 140 °C |
| storage temp. | 2-8°C |
| Water Solubility | Soluble in water |
| form | powder to crystal |
| color | Pale white powder |
| Merck | 14,7650 |
| BRN | 4167152 |
| Hydrophilic-Lipophilic Balance (HLB) | 20 |
| CAS DataBase Reference | 143-18-0(CAS DataBase Reference) |
| EPA Substance Registry System | Potassium oleate (143-18-0) |
Safety information for Potassium oleate
| Signal word | Warning |
| Pictogram(s) |
 Exclamation Mark Irritant GHS07  Environment GHS09 |
| GHS Hazard Statements |
H315:Skin corrosion/irritation H319:Serious eye damage/eye irritation H335:Specific target organ toxicity, single exposure;Respiratory tract irritation H400:Hazardous to the aquatic environment, acute hazard |
| Precautionary Statement Codes |
P273:Avoid release to the environment. P302+P352:IF ON SKIN: wash with plenty of soap and water. P305+P351+P338:IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. Continuerinsing. |
Computed Descriptors for Potassium oleate
| InChIKey | MLICVSDCCDDWMD-KVVVOXFISA-M |
New Products
Methyl (R)-1-Boc-4,4-difluoropyrrolidine-2-carboxylate 2,2-Difluoropropylamine hydrochloride tert-butyl 3-bromoazetidine-1-carboxylate (R)-1-Boc-3-hydroxypyrrolidine DIFLUOROACETIC ANHYDRIDE 2,2-Difluoropropionic acid Diallylamine, 99% Calcium hydroxide, 95% Aluminum oxide, basic 2-Bromophenylacetonitrile, 97% L-tert-Leucine,97% N-Hydroxy-2-methylpropanimidamide 4-(3,4-Dichlorophenyl)-3,4-Dihydro-N-Methyl-1-(2H)-Naphthalenimine (Schiff Base) 2-AMINO-3,5-DIBROMO BENZALDEHYDE [ADBA] L-Glutamic Acid Dimethyl Ester Hcl 10-Methoxy-5H-dibenz[b,f]azepine 5-Cyanophthalide N, N-Carbonyldiimidazole (CDI) Dibenzoyl Peroxide Titanium Dioxide 2-(Methylthio) Benzonitrile Sodium Acetate Anhydrous Allopurinol 1,5-DibromopentaneRelated products of tetrahydrofuran
You may like
-
 143-18-0 Potassium oleate 98%View Details
143-18-0 Potassium oleate 98%View Details
143-18-0 -
 143-18-0 99%View Details
143-18-0 99%View Details
143-18-0 -
 Potassium oleate powder CAS 143-18-0View Details
Potassium oleate powder CAS 143-18-0View Details
143-18-0 -
 Potassium Oleate CAS 143-18-0View Details
Potassium Oleate CAS 143-18-0View Details
143-18-0 -
 Potassium oleate, paste CAS 143-18-0View Details
Potassium oleate, paste CAS 143-18-0View Details
143-18-0 -
 Potassium oleate CAS 143-18-0View Details
Potassium oleate CAS 143-18-0View Details
143-18-0 -
 Ethyl-2-Chloroacetoacetate 609-15-4View Details
Ethyl-2-Chloroacetoacetate 609-15-4View Details
609-15-4 -
 609-15-4View Details
609-15-4View Details
609-15-4
