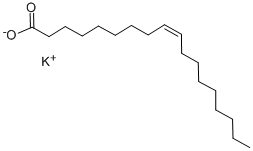
Sodium oleate
Synonym(s):cis-9-Octadecenoic acid sodium salt;Oleic acid sodium salt
- CAS NO.:143-19-1
- Empirical Formula: C18H33NaO2
- Molecular Weight: 304.44
- MDL number: MFCD00004438
- EINECS: 205-591-0
- SAFETY DATA SHEET (SDS)
- Update Date: 2025-01-27 09:38:02
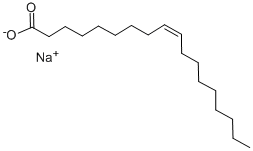
What is Sodium oleate?
Chemical properties
Sodium oleate is a white solid with a mild odor resembling tallow. It is capable of dissolving in water but may undergo partial decomposition in the process. In addition, it is soluble in alcohol and can produce froth or foam when shaken with a water solution (soap). Sodium oleate is produced by reaction of NaOH and oleic acid (in alcoholic solution) and evaporating. It can be used as a source of oleate.
The Uses of Sodium oleate
sodium oleate is a mild cleansing and foaming agent generally used in soaps. It is derived from natural fats and oils.
The Uses of Sodium oleate
preparation of Turkey red oil, soft soap and other oleates; in polishing Compounds; waterproofing textiles, oiling wool; manufacture of driers; thickening lubricating oils. Pharmaceutic aid (solvent). The barium salt in rodent extermination.
The Uses of Sodium oleate
Sodium Oleate is the sodium salt of oleic acid. it functions as a binder, emulsifier, and anticaking agent.
Definition
ChEBI: Sodium oleate is an organic molecular entity.
General Description
Light tan solid with a slight tallow-like odor. Sinks and mixes slowly with water.
Air & Water Reactions
Water soluble. Gives basic aqueous solution.
Reactivity Profile
Salts, basic, such as SODIUM OLEATE, are generally soluble in water. The resulting solutions contain moderate concentrations of hydroxide ions and have pH's greater than 7.0. They react as bases to neutralize acids. These neutralizations generate heat, but less or far less than is generated by neutralization of the bases in reactivity group 10 (Bases) and the neutralization of amines. They usually do not react as either oxidizing agents or reducing agents but such behavior is not impossible.
Health Hazard
Inhalation of dust causes irritation of nose and throat, coughing, and sneezing. Ingestion causes mild irritation of mouth. Contact with eyes causes irritation.
Biochem/physiol Actions
Oleic acid increases hepatic secretion of apolipoprotein B100 in hepatocyte cell lines and in mice . Oleic acid inhibits apolipoprotein B100 secretion at higher physiologic doses .
Safety Profile
Poison by intravenous route. Migrates to food from packaging materials. Combustible when exposed to heat or flame. When heated to decomposition it emits toxic fumes of Na2O
Purification Methods
It crystallises from EtOH and is dried in an oven at 100o. [Beilstein 2 H 465, 2 I 201, 2 II 434, 2 III 1405, 2 IV 1645.]
Properties of Sodium oleate
| Melting point: | 232-235 °C (lit.) |
| storage temp. | -20°C |
| solubility | Methanol (Slightly) |
| form | Powder |
| color | White to slightly yellow |
| Odor | slt tallow odor |
| Water Solubility | soluble H2O, partially decomposes; soluble alcohol [HAW93] |
| Merck | 14,6828 |
| BRN | 4046357 |
| Hydrophilic-Lipophilic Balance (HLB) | 18 |
| Dielectric constant | 2.7(20℃) |
| Stability: | Stable. Incompatible with strong oxidizing agents. |
| InChI | InChI=1S/C18H34O2.Na/c1-2-3-4-5-6-7-8-9-10-11-12-13-14-15-16-17-18(19)20;/h9-10H,2-8,11-17H2,1H3,(H,19,20);/q;+1/p-1/b10-9-; |
| CAS DataBase Reference | 143-19-1(CAS DataBase Reference) |
| EPA Substance Registry System | Sodium oleate (143-19-1) |
Safety information for Sodium oleate
| Signal word | Warning |
| Pictogram(s) |
 Exclamation Mark Irritant GHS07 |
| GHS Hazard Statements |
H412:Hazardous to the aquatic environment, long-term hazard |
| Precautionary Statement Codes |
P273:Avoid release to the environment. |
Computed Descriptors for Sodium oleate
| InChIKey | BCKXLBQYZLBQEK-KVVVOXFISA-M |
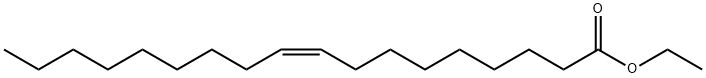
| SMILES | C(CCCCCC([O-])=O)C/C=C\CCCCCCCC.[Na+] |
New Products
3-Iodophenylacetic acid 3-Pyridineacetonitrile, α-hydroxy- 2-Propanamine, 1-chloro-, hydrochloride (9CI) 3-(hexyloxy)-4-(pyridin-3-yl)-1,2,5-thiadiazole 2-Hexyn-1-ol Dibenzo-18-crown-6 Nickel(II) perchlorate hexahydrate, 98% 4-Bromophenylacetonitrile, 95% 3-Bromo-4-fluoroaniline, 97% Sodium tetraborate decahydrate, 98% Palladium(II) acetate, trimer, Pd 99% 4-Bromo-2-chlorotoluene, 97% N N Dimethylformamide Dimethyl Acetal (Dmf Dma) 2,3-Dichloro Benzoyl Cyanide [Side Chain] Bis(2-Chloroethyl) Amine Hydrochloride L-Glutamic Acid Diethyl Ester Hydrochloride 5-(Difluoromethoxy)-2-Mercaptobenzimidazole 1-Ethyl-3-(3-Dimethylaminopropyl)-Carbodiimide Hydrochloride [EDC Hcl] 1,4-Napthoquinone Bromoiodomethane Sodium Bicarbonate Methylene Dichloride (MDC) Ethyl Acetate Indole-3-Carbinol (I3C)Related products of tetrahydrofuran
You may like
-
 Sodium oleate powder CAS 143-19-1View Details
Sodium oleate powder CAS 143-19-1View Details
143-19-1 -
 Sodium Oleate CAS 143-19-1View Details
Sodium Oleate CAS 143-19-1View Details
143-19-1 -
 Sodium oleate 99% CAS 143-19-1View Details
Sodium oleate 99% CAS 143-19-1View Details
143-19-1 -
 Sodium oleate 95.00% CAS 143-19-1View Details
Sodium oleate 95.00% CAS 143-19-1View Details
143-19-1 -
 Sodium oleate, paste CAS 143-19-1View Details
Sodium oleate, paste CAS 143-19-1View Details
143-19-1 -
 SODIUM OLEATE Pure CAS 143-19-1View Details
SODIUM OLEATE Pure CAS 143-19-1View Details
143-19-1 -
 Sodium oleate CAS 143-19-1View Details
Sodium oleate CAS 143-19-1View Details
143-19-1 -
 Sodium oleate CAS 143-19-1View Details
Sodium oleate CAS 143-19-1View Details
143-19-1
