Oleamide
Synonym(s):cis-9,10-Octadecenoamide;cis-9-Octadecenamide;Oleic acid amide
- CAS NO.:301-02-0
- Empirical Formula: C18H35NO
- Molecular Weight: 281.48
- MDL number: MFCD00053638
- EINECS: 206-103-9
- SAFETY DATA SHEET (SDS)
- Update Date: 2025-12-26 08:49:36
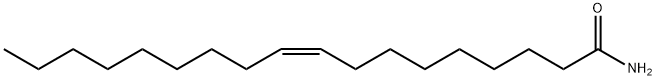
What is Oleamide?
Description
OOleamide (301-02-0) was originally identified in the cerebrospinal fluid of sleep-deprived cats acting as an inducer of physiological sleep in animals.1 Displays agonist activity at cannabinoid CB1 receptors (Ki=8.13 μM).2 Activates PPARγ.3 Produces vasodilator effects in rats.4 Displays neuroprotective effects5 and attenuates sepsis-induced intestinal injury6.
Chemical properties
White Powder
The Uses of Oleamide
Oleamide has been used as a supplement in glucose and galactose media to prevent the rescue of galactose-induced Leigh syndrome (LS).
The Uses of Oleamide
A brain lipid that induces physiological sleep at nanomolar quantities when injected into rats. This lipid may represent a new class of biological signaling molecules.
What are the applications of Application
Oleamide is a CB1, 5-HT2A and 2C receptor agonist
Definition
ChEBI: A fatty amide derived from oleic acid.
General Description
Oleamide?is a lipid or a brain fatty acid, that is found in the CSF (cerebrospinal fluid). It is originally obtained from the cerebrospinal fluid of cats, that are sleep-deprived.
Biological Activity
Endogenous sleep-inducing lipid. Acts as an agonist at the CB 1 cannabinoid receptor (EC 50 = 1.64 μ M). Also appears to potentiate the actions of 5-HT on 5-HT 2A and 2C receptors, and act via an allosteric regulatory site on 5-HT 7 receptors.
Biochem/physiol Actions
Sleep-inducing brain lipid, which allosterically modulates GABAA receptors and potentiates 5-HT7 serotonin receptor responses. Selective endogenous agonist of rat and human CB1 cannabinoid receptor.
Synthesis
Oleamide can be synthesized by ammonolysis of fatty acid or esters with ammonia gas at high pressure.
Storage
Store at +4°C
References
Boger et al. (1998), Oleamide: an endogenous sleep-inducing lipid and prototypical member of a new class of biological signaling molecules; Curr. Pharm. Des., 4 303 Leggett et al. (2004), Oleamide is a selective endogenous agonist of rat and human CB1 cannabinoid receptors; Br. J. Pharmacol., 141 253 Dionisi et al. (2012), Oleamide activates peroxisome proliferator-activated receptor gamma (PPARγ) in vitro; Lipids Health Dis., 11 51 Hernandez-Diaz et al. (2020), Effects of Oleamide on the Vasomotor Responses in the Rat; Cannabis Cannabinoid Res. 5 42 Maya-Lopez et al. (2020), A Cannabinoid Receptor-Mediated Mechanism Participates in the Neuroprotective Effects of Oleamide Against Excitotoxic Damage in Rat Brain Synaptosomes and Cortical Slices; Neurotox. Res., 37 126 Zou et al. (2019), Cx43 Inhibition Attenuates Sepsis-Induced Intestinal Injury via Downregulating ROS Transfer and the Activation of the JNK1/Sirt1/FoxO3a Signaling Pathway; Mediators Inflamm., 2019 7854389
Properties of Oleamide
| Melting point: | 70°C |
| Boiling point: | 433.3±24.0 °C(Predicted) |
| Density | 0.94 g/cm3 |
| storage temp. | -20°C |
| solubility | Soluble in chloroform (50 mg/ml), ethanol (100 mM), DMSO (~14 mg/ml), and DMF (~14 mg/ml) |
| form | neat |
| pka | 16.61±0.40(Predicted) |
| form | Solid |
| color | White to off-white |
| Water Solubility | Insoluble in water. |
| Stability: | Stable for 2 years from date of purchase as supplied. Solutions in DMSO or ethanol may be stored at -20°C for up to 1 month. |
| CAS DataBase Reference | 301-02-0(CAS DataBase Reference) |
| NIST Chemistry Reference | 9-Octadecenamide, (z)-(301-02-0) |
| EPA Substance Registry System | Oleamide (301-02-0) |
Safety information for Oleamide
| Signal word | Warning |
| Pictogram(s) |
 Exclamation Mark Irritant GHS07 |
| GHS Hazard Statements |
H413:Hazardous to the aquatic environment, long-term hazard |
| Precautionary Statement Codes |
P273:Avoid release to the environment. P501:Dispose of contents/container to..… |
Computed Descriptors for Oleamide
| InChIKey | FATBGEAMYMYZAF-KTKRTIGZSA-N |
New Products
4,4-Difluoropiperidine hydrochloride tert-butyl 9-methoxy-3-azaspiro[5.5]undecane-3-carboxylate Indole Methyl Resin N-Isopropylurea N,N-Dicyclohexylcarbodiimide(DCC) MELDRUMS ACID 5-METHYLISOXAZOLE-4-CARBOXYLIC ACID Magnessium Bis glycinate Zinc ascorbate 1-bromo-2-butyne 2-acetamidophenol 9(10H)-anthracenone Erythrosin B, 4-Piperidinopiperidine 2-((4-morpholinophenylamino) (methylthio) methylene) malononitrile 2,4-dihydroxybenzaldehyde 3-(4-morpholinophenylamino)-5-amino-1H-pyrazole-4-carbonitrile Methyl 2-methylquinoline-6-carboxylate 2,6-dichloro-4-nitropyridine 4-Bromo-2-chlorobenzonitrile 2-(benzylamino)acetic acid hydrochloride 4-(tert-Butoxycarbonylamino)but- 2-ynoic acid 3,4-dihydro-2H-benzo[b][1,4]dioxepine 1-Phenyl-1-cycloprppanecarboxylicacidRelated products of tetrahydrofuran
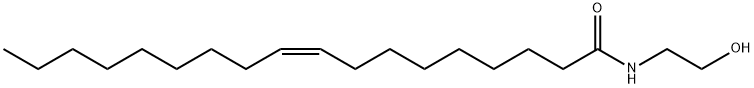
![disodium C-[2-[2-[(1-oxooctadec-9-enyl)amino]ethoxy]ethyl] sulphonatosuccinate](https://img.chemicalbook.in/)
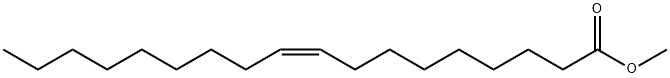
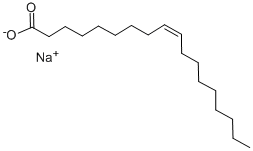
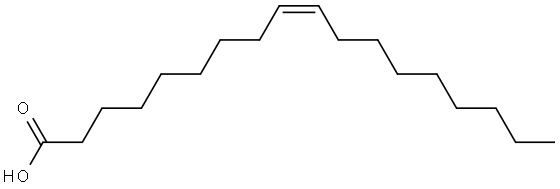
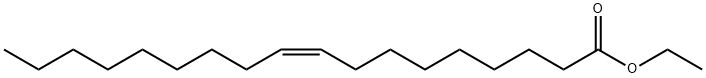
You may like
-
 Oleamide CAS 301-02-0View Details
Oleamide CAS 301-02-0View Details
301-02-0 -
 Oleamide 65% CAS 301-02-0View Details
Oleamide 65% CAS 301-02-0View Details
301-02-0 -
 Oleamide CAS 301-02-0View Details
Oleamide CAS 301-02-0View Details
301-02-0 -
 Oleamide (301-02-0) (C18H35NO), Purity: 96-98%View Details
Oleamide (301-02-0) (C18H35NO), Purity: 96-98%View Details
301-02-0 -
 3-(4-amino-1-oxoisoindolin-2-yl)-1-methylpiperidine-2,6-dione 98%View Details
3-(4-amino-1-oxoisoindolin-2-yl)-1-methylpiperidine-2,6-dione 98%View Details -
 20677-73-0 (2,2-diethoxyethyl)methylamine 98%View Details
20677-73-0 (2,2-diethoxyethyl)methylamine 98%View Details
20677-73-0 -
 3-(4-(hydroxyamino)-1-oxoisoindolin-2-yl)piperidine-2,6-dione 98%View Details
3-(4-(hydroxyamino)-1-oxoisoindolin-2-yl)piperidine-2,6-dione 98%View Details -
 57381-49-4 2-bromo-4-chlorobenzonitrile 98%View Details
57381-49-4 2-bromo-4-chlorobenzonitrile 98%View Details
57381-49-4
