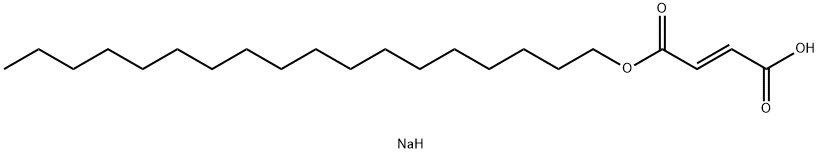
Sodium Stearyl Fumarate
Synonym(s):(E)-2-Butenedioic acid monooctadecyl ester sodium salt;Sodium octadecyl fumarate;Sodium stearyl fumarate
- CAS NO.:4070-80-8
- Empirical Formula: C22H41NaO4
- Molecular Weight: 392.56
- MDL number: MFCD07782099
- EINECS: 223-781-1
- SAFETY DATA SHEET (SDS)
- Update Date: 2025-01-27 09:38:02
What is Sodium Stearyl Fumarate?
Description
Sodium Stearyl Fumarate (SSF) is used as a lubricant in tableting to reduce the friction between the tablet and the die wall and to prevent adhesion of the material to punches or the die wall in the pharmaceutical industries. It is used also as a lubricant in the manufacturing of capsules and other oral dosage forms. Sodium stearyl fumarate is also used in food production as a dough conditioner, a conditioning agent, and a stabilizing agent.
Chemical properties
White or off-white crystalline powder
Chemical properties
Sodium stearyl fumarate is a fine, white powder with agglomerates of flat, circular-shaped particles.
The Uses of Sodium Stearyl Fumarate
Sodium Stearyl Fumarate is a dough conditioner and conditioning agent that is a white powder practically insoluble in water. It is used as a dough conditioner in yeast-raised baked goods. It is used as a conditioning agent in dehydrated potatoes. It also functions as a maturing and bleaching agent.
The Uses of Sodium Stearyl Fumarate
cationic transfection agent, bile acid sequestrant, vaccine adjuvant, protein stabilizer
The Uses of Sodium Stearyl Fumarate
Binder
Production Methods
Stearyl alcohol is reacted with maleic anhydride. The product of this reaction then undergoes an isomerization step followed by salt formation to produce sodium stearyl fumarate.
Pharmaceutical Applications
Sodium stearyl fumarate is used as a lubricant in capsule and tablet formulations at 0.5–2.0% w/w concentration. It is also used in certain food applications.
Safety
Sodium stearyl fumarate is used in oral pharmaceutical formulations
and is generally regarded as a nontoxic and nonirritant
material.
Metabolic studies of sodium stearyl fumarate in the rat and dog
indicated that approximately 80% was absorbed and 35% was
rapidly metabolized. The fraction absorbed was hydrolyzed to stearyl alcohol and fumaric acid, with the stearyl alcohol further
oxidized to stearic acid. In the dog, sodium stearyl fumarate that
was not absorbed was excreted unchanged in the feces within 24
hours.
Stearyl alcohol and stearic acid are naturally occurring
constituents in various food products, while fumaric acid is a
normal constituent of body tissue. Stearates and stearyl citrate have
been reviewed by the WHO and an acceptable daily intake for
stearyl citrate has been set at up to 50 mg/kg body-weight. The
establishment of an acceptable daily intake for stearates and
fumaric acid was thought unnecessary.
Disodium fumarate has been reported to have a toxicity not
greatly exceeding that of sodium chloride.
storage
At ambient temperature, sodium stearyl fumarate is stable for up to
3 years when stored in amber glass bottles with polyethylene screw
caps.
The bulk material should be stored in a well-closed container in a
cool, dry place.
Incompatibilities
Sodium stearyl fumarate is reported to be incompatible with chlorhexidine acetate.
Regulatory Status
GRAS listed. Permitted by the FDA for direct addition to food for human consumption as a conditioning or stabilizing agent in various bakery products, flour-thickened foods, dehydrated potatoes, and processed cereals up to 0.2–1.0% by weight of the food. Included in nonparenteral medicines licensed in the UK. Included in the FDA Inactive Ingredients Database (oral capsules and tablets). Included in the Canadian List of Acceptable Non-medicinal Ingredients.
References
[1] Arne W. Hölzer and John Sjögren, Evaluation of sodium stearyl fumarate as a tablet lubricant, International Journal of Pharmaceutics, 1979, vol. 2, 145-153
[2] Holger Zorn and Peter Czermak, Biotechnology of Food and Feed Additives, 2014
[3] https://www.fda.gov
Properties of Sodium Stearyl Fumarate
| Melting point: | >196°C (dec.) |
| storage temp. | Inert atmosphere,Room Temperature |
| solubility | Practically insoluble in water, slightly soluble in methanol, practically insoluble in acetone and in anhydrous ethanol. |
| form | neat |
| form | Solid |
| color | White to Off-White |
Safety information for Sodium Stearyl Fumarate
| Signal word | Warning |
| Pictogram(s) |
 Exclamation Mark Irritant GHS07 |
| GHS Hazard Statements |
H302:Acute toxicity,oral H315:Skin corrosion/irritation H319:Serious eye damage/eye irritation H335:Specific target organ toxicity, single exposure;Respiratory tract irritation |
| Precautionary Statement Codes |
P261:Avoid breathing dust/fume/gas/mist/vapours/spray. P280:Wear protective gloves/protective clothing/eye protection/face protection. P301+P312:IF SWALLOWED: call a POISON CENTER or doctor/physician IF you feel unwell. P302+P352:IF ON SKIN: wash with plenty of soap and water. P305+P351+P338:IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. Continuerinsing. |
Computed Descriptors for Sodium Stearyl Fumarate
| InChIKey | GKCVIRALYIXVLE-RZLHGTIFSA-M |
Sodium Stearyl Fumarate manufacturer
AKASH PHARMA EXPORTS
Attar Global
Organo Source
Bazayan & Co.
New Products
3-Iodophenylacetic acid 3-Pyridineacetonitrile, α-hydroxy- 2-Propanamine, 1-chloro-, hydrochloride (9CI) 3-(hexyloxy)-4-(pyridin-3-yl)-1,2,5-thiadiazole 2-Hexyn-1-ol Dibenzo-18-crown-6 Nickel(II) perchlorate hexahydrate, 98% 4-Bromophenylacetonitrile, 95% 3-Bromo-4-fluoroaniline, 97% Sodium tetraborate decahydrate, 98% Palladium(II) acetate, trimer, Pd 99% 4-Bromo-2-chlorotoluene, 97% N N Dimethylformamide Dimethyl Acetal (Dmf Dma) 2,3-Dichloro Benzoyl Cyanide [Side Chain] Bis(2-Chloroethyl) Amine Hydrochloride L-Glutamic Acid Diethyl Ester Hydrochloride 5-(Difluoromethoxy)-2-Mercaptobenzimidazole 1-Ethyl-3-(3-Dimethylaminopropyl)-Carbodiimide Hydrochloride [EDC Hcl] 1,4-Napthoquinone Bromoiodomethane Sodium Bicarbonate Methylene Dichloride (MDC) Ethyl Acetate Indole-3-Carbinol (I3C)Related products of tetrahydrofuran


![Benzenebutanoicacid,b-[[(1,1-diMethylethoxy)carbonyl]aMino]-2,4,5-trifluoro-,Methylester,(bR)-](https://img.chemicalbook.in/CAS/20150408/GIF/881995-73-9.gif)
You may like
-
 4070-80-8 99%View Details
4070-80-8 99%View Details
4070-80-8 -
 Sodium Stearyl Fumarate 99%View Details
Sodium Stearyl Fumarate 99%View Details -
 Sodium stearyl fumarate 95% CAS 4070-80-8View Details
Sodium stearyl fumarate 95% CAS 4070-80-8View Details
4070-80-8 -
 Sodium stearyl fumarate 96% CAS 4070-80-8View Details
Sodium stearyl fumarate 96% CAS 4070-80-8View Details
4070-80-8 -
 Sodium Stearyl Fumarate CAS 4070-80-8View Details
Sodium Stearyl Fumarate CAS 4070-80-8View Details
4070-80-8 -
 Sodium stearyl fumarate CAS 4070-80-8View Details
Sodium stearyl fumarate CAS 4070-80-8View Details
4070-80-8 -
 Sodium stearyl fumarate CAS 4070-80-8View Details
Sodium stearyl fumarate CAS 4070-80-8View Details
4070-80-8 -
 Sodium Stearyl Fumarate CAS 4070-80-8View Details
Sodium Stearyl Fumarate CAS 4070-80-8View Details
4070-80-8
