Ponatinib Hydrochloride
- CAS NO.:1114544-31-8
- Empirical Formula: C29H28ClF3N6O
- Molecular Weight: 569.03
- MDL number: MFCD28656903
- EINECS: 1592732-453-0
- SAFETY DATA SHEET (SDS)
- Update Date: 2024-07-25 20:04:46
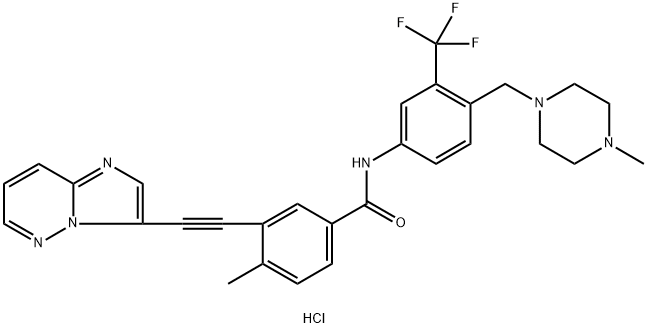
What is Ponatinib Hydrochloride?
Description
In December 2012, the US FDA approved ponatinib (also referred to as AP 24534) for the treatment of adult patients with chronic phase, accelerated phase, or blast phase chronicmyeloid leukemia (CML). Ponatinib is a pan-Bcr–Abl TKI that blocks both the native (IC50=0.4 nM) and Bcr– AblT315I mutated kinases (IC50=2.0 nM) in addition to othermutated kinases in CML patients. In the Ba/F3 cell proliferation assay, ponatinib inhibits ABL and the T315I Abl mutant with IC50s of 1.2 and 8.8 nM, respectively. Ponatinib was identified by a structure-based drug design approach. Ponatinib binds to the kinase domain in a DFG-out conformation; the ethynyl moiety helps the inhibitor evade the mutant gatekeeper isoleucine residue at position 315. In addition to Abl and the T315I mutant of Abl, ponatinib inhibits VEGFR, PDGFR, FGFR, SRC, KIT, RET, TIE2, FLT3, and EPH receptors at concentrations ranging from0.1 to 20 nM.
Originator
Ariad (United States)
The Uses of Ponatinib Hydrochloride
Ponatinib is a tyrosine kinase inhibitors (TKI) and used to treat chronic myeloid leukemia (CML) (1,2,3). Ponatinib is also used in combination with other drugs, such as forskolin, to combat TKI resistance in patient with CML (3), targeted drug in small-cell lung cancer. Potent FAK inhibitor. FGFR/VEGFR/Bcr-Abl inhibitor It is a COVID19-related research product.
What are the applications of Application
AP 24534 HCl is a salt form of AP 24534 which is a tyrosine kinase inhibitor
Definition
ChEBI: Ponatinib hydrochloride is the hydrochloride salt of ponatinib. It is a potent pan inhibitor of tyrosine kinases, active in all single resistance ABL kinase mutations including the T315l mutation. It is approved for the treatment of chronic myeloid leukemia. It has a role as an antineoplastic agent and a tyrosine kinase inhibitor. It contains a ponatinib(1+).
brand name
Iclusig
Clinical Use
Ponatinib hydrochloride (Iclusig ®), previously known as AP24534, is a multi-targeted tyrosine kinase inhibitor approved in the US as an oral treatment for resistant or intolerant chronic myeloid leukemia (CML) and Philadelphia chromosome-positive (Ph+) acute lymphoblastic leukemia (ALL). Ponatinib hydrochloride was designed for treatment of tumors containing the T351I mutation which are present in some forms of CML and resistant to traditional therapies such as imatinib. Ponatinib hydrochloride was developed by Ariad Pharmaceauticals, and operates by a similar mechanism of action as other tyrosine kinase inhibitors, inhibiting the enzymatic activity of BCR-ABL, an abnormal tyrosine kinase responsible for unregulated and excess white blood cell production by bone marrow. However, the ability of ponatinib hydrochloride to target isoforms of the BCR-ABL gene typically leading to resistance in other known tyrosine kinase inhibitors provides an alternate form of therapy not previously available.
Synthesis
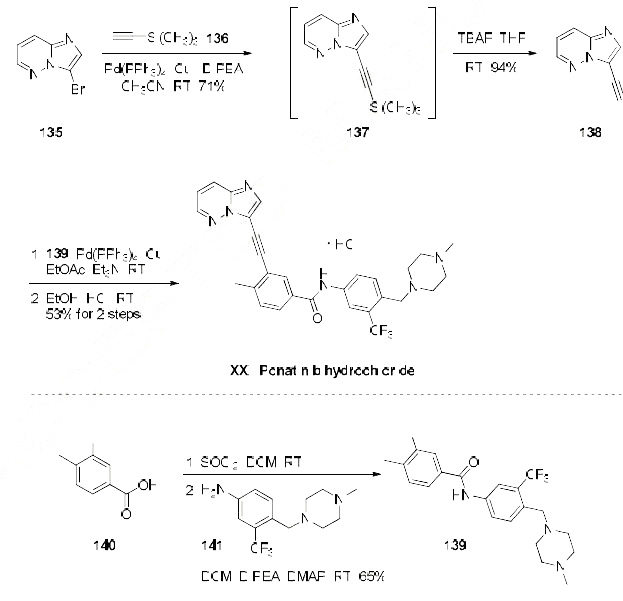
A significant amount of research has been devoted towards identification of a manufacturing synthesis of ponatinib hydrochloride. A majority of methods rely on two key Sonagashira couplings to generate the imidazo[1,2-b]pyridazin-3-ylethynyl framework. The most likely process scale method begins with 3-bromo-imidazo[1,2-b]pyridazine (135) (the Scheme). Direct Sonogashira coupling of (135) with ethynyltrimethylsilane (136) in the presence of Pd(PPh3)4 and CuI, followed by treatment with TBAF/THF led to the desired alkynyl imidazo[1,2-b]pyridazine 138 in 71 and 94% yields, respectively. Alkyne 138 was then coupled under similar Sonogashira conditions with functionalized aryl iodide 139 (generated in two steps from 3-iodo-4-methylbenzoic acid (140) and commercially available piperazinyl aniline 141) providing ponatinib free base, which was then immediately treated with EtOH/HCl at room temperature to ultimately furnish ponatinib hydrochloride (XXI).
Properties of Ponatinib Hydrochloride
| storage temp. | -20°C |
| solubility | DMF: 2 mg/ml,DMSO: 11 mg/ml,Ethanol: Slightly soluble |
| form | A solid |
| color | Off-white to light yellow |
Safety information for Ponatinib Hydrochloride
Computed Descriptors for Ponatinib Hydrochloride
New Products
Indole Methyl Resin tert-butyl 9-methoxy-3-azaspiro[5.5]undecane-3-carboxylate Boc-His(Boc)-OH 2-CTC Resin 4-Chloro-7-tosy1-7Hpyrrolo[2,3-d]pyrimidine 5,7-Dibromo-1H-indole 2,5-dichloro-N-hydroxy-4,6-dimethylpyridine-3-carboximidamide 2,2-Dimethoxy-7-azaspiro[3.5]nonane hydrochloride 4-chloromethyl-5-methyl-1,3-dioxol-2-one (DMDO-Cl) R-2-BENZYLOXY PROPIONIC ACID 1,1’-CARBONYLDIIMIDAZOLE 1,1’-CARBONYLDI (1,2-4 TRIAZOLE) N-METHYL INDAZOLE-3-CARBOXYLIC ACID 4-((2-hydroxyethyl)thio)benzoic acid 1-(TERT-BUTOXYCARBONYL)-2-PYRROLIDINONE Methyl 6-methylnicotinate 3-Pyridineacrylic acid tert-Butyl carbazate TETRAHYDRO-2H-PYRAN-3-OL 2-((4-morpholinophenylamino) (methylthio) methylene) malononitrile 3-(4-morpholinophenylamino)-5-amino-1H-pyrazole-4-carbonitrile 2,4-dihydroxybenzaldehyde 1,3-Diethyl-1,3-Diphenylurea Methyl 2-methylquinoline-6-carboxylateRelated products of tetrahydrofuran
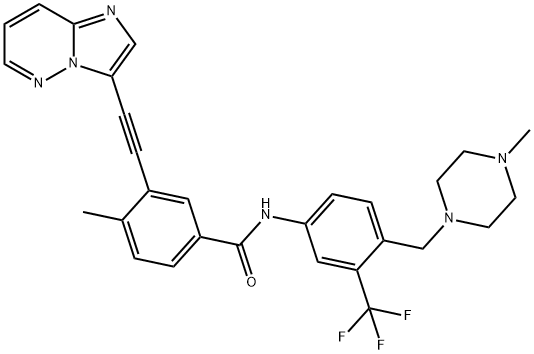
You may like
-
 1114544-31-8 Ponatinib hydrochloride 98%View Details
1114544-31-8 Ponatinib hydrochloride 98%View Details
1114544-31-8 -
 Ponatinib (HCl salt) >95% CAS 1114544-31-8View Details
Ponatinib (HCl salt) >95% CAS 1114544-31-8View Details
1114544-31-8 -
 Pyridine 99.5% HPLC /UV SpectroscopyView Details
Pyridine 99.5% HPLC /UV SpectroscopyView Details
110-86-1 -
 Piperazine Spot supply, best priceView Details
Piperazine Spot supply, best priceView Details
110-85-0 -
 Dibutyl PhthalateView Details
Dibutyl PhthalateView Details
84-74-2 -
 Imidazole Spot supply, competitive priceView Details
Imidazole Spot supply, competitive priceView Details
288-32-4 -
 Octadecyl 3-(3,5-di-tert-butyl-4-hydroxyphenyl)propionate 98% (GC)View Details
Octadecyl 3-(3,5-di-tert-butyl-4-hydroxyphenyl)propionate 98% (GC)View Details
2082-79-3 -
 Thiourea 99% ARView Details
Thiourea 99% ARView Details
62-56-6
