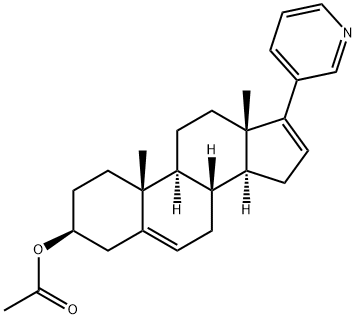
Galeterone
Synonym(s):3β-Hydroxy-17-(1H-benzimidazole-1-yl)androsta-5,16-diene;TOK-001;VN/124-1;VN-124-1
- CAS NO.:851983-85-2
- Empirical Formula: C26H32N2O
- Molecular Weight: 388.55
- MDL number: MFCD16660907
- EINECS: 806-537-4
- SAFETY DATA SHEET (SDS)
- Update Date: 2025-12-26 16:58:18
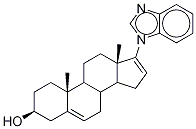
What is Galeterone?
Description
Galeterone (TOK-001) is a small molecule oral drug that disrupts AR signaling by a novel triple mechanism: it potently and selectively inhibits CYP17 lyase, potently antagonizes AR and decreases AR protein levels (wild-type and mutant), leading to antitumor activity. Galeterone has shown significant anti-tumour activity with a well-tolerated safety profile in patients with CRPC in phase I and II clinical studies.
The Uses of Galeterone
Galeterone is an androgen receptor antagonist and CYP17A1 enzyme inhibitor. It has been used in trials studying the treatment of Prostate Cancer.
What are the applications of Application
Galeterone is an antagonist to LNCaP AR
Definition
ChEBI: Galeterone is a 3-hydroxy steroid. It has a role as an androgen.
Biological Activity
The cytochrome P450 (CYP) isoform CYP17 is also known as steroid 17α-hydroxylase/17,20 lyase because it catalyzes both 17α-hydroxylase and 17,20 lyase reactions in the synthesis of steroids, including androgens, estrogens, glucocorticoids, and mineralocorticoids. Galeterone is a CYP17 inhibitor (IC50 = 300 nM) that has been shown to competitively block synthetic androgen binding (EC50 = 845 nM) and to antagonize the androgen receptor in transcriptional activation assays. Galeterone can inhibit the growth of castration-resistant prostate cancer cells with an IC50 value of 2.9 μM and demonstrates synergy with everolimus or gefitinib for growth inhibition.
Storage
Store at -20°C
Mode of action
Galeterone is an orally bioavailable small-molecule androgen receptor modulator and CYP17 lyase inhibitor with potential antiandrogen activity. Galeterone exhibits three distinct mechanisms of action: 1) as an androgen receptor antagonist, 2) as a CYP17 lyase inhibitor and 3) by decreasing overall androgen receptor levels in prostate cancer tumors, all of which may result in a decrease in androgen-dependent growth signaling. Localized to the endoplasmic reticulum (ER), the cytochrome P450 enzyme CYP17 (P450C17 or CYP17A1) exhibits both 17alpha-hydroxylase and 17,20-lyase activities, and plays a key role in the steroidogenic pathway that produces progestins, mineralocorticoids, glucocorticoids, androgens, and estrogens.
References
[1]. devore n m, & scott e e. structures of cytochrome p450 17a1 with prostate cancer drugs abiraterone and tok-001. nature, 2012, 482: 116-119.
[2]. mallik i, davila m, tapia t, et al. androgen regulates cdc6 transcription through interactions between androgen receptor and e2f transcription factor in prostate cancer cells. biochimica et biophysica acta (bba) - molecular cell research, 2008,1783:1737-1744.
[3]. bruno r d, vasaitis t s, gediya l. k, et al. synthesis and biological evaluations of putative metabolically stable analogs of vn/124-1 (tok-001): head to head anti-tumor efficacy evaluation of vn/124-1 (tok-001) and abiraterone in lapc-4 human prostate cancer xenograft model. steroids, 2011,76: 1268-1279.
Properties of Galeterone
| Melting point: | 189-190℃ |
| Boiling point: | 564.5±60.0 °C(Predicted) |
| Density | 1.28 |
| storage temp. | -20°C Freezer |
| solubility | Chloroform (Slightly), Ethyl Aceatae (Slightly, Heated) |
| form | Solid |
| pka | 14.71±0.70(Predicted) |
| color | White to Off-White |
Safety information for Galeterone
Computed Descriptors for Galeterone
| InChIKey | PAFKTGFSEFKSQG-MRFMOSGMNA-N |
| SMILES | C1C[C@]2(C)C3CC[C@]4(C)C([n]5cnc6ccccc56)=CCC4C3CC=C2C[C@H]1O |&1:2,7,27,r| |
New Products
4,4-Difluoropiperidine hydrochloride tert-butyl 9-methoxy-3-azaspiro[5.5]undecane-3-carboxylate Indole Methyl Resin N-Isopropylurea N,N-Dicyclohexylcarbodiimide(DCC) MELDRUMS ACID 5-METHYLISOXAZOLE-4-CARBOXYLIC ACID Magnessium Bis glycinate Zinc ascorbate 1-bromo-2-butyne 2-acetamidophenol 9(10H)-anthracenone Erythrosin B, 4-Piperidinopiperidine 2-((4-morpholinophenylamino) (methylthio) methylene) malononitrile 2,4-dihydroxybenzaldehyde 3-(4-morpholinophenylamino)-5-amino-1H-pyrazole-4-carbonitrile Methyl 2-methylquinoline-6-carboxylate 2,6-dichloro-4-nitropyridine 4-Bromo-2-chlorobenzonitrile 2-(benzylamino)acetic acid hydrochloride 4-(tert-Butoxycarbonylamino)but- 2-ynoic acid 3,4-dihydro-2H-benzo[b][1,4]dioxepine 1-Phenyl-1-cycloprppanecarboxylicacidRelated products of tetrahydrofuran
You may like
-
 Galeterone 98% (HPLC) CAS 851983-85-2View Details
Galeterone 98% (HPLC) CAS 851983-85-2View Details
851983-85-2 -
 Galeterone CAS 851983-85-2View Details
Galeterone CAS 851983-85-2View Details
851983-85-2 -
 3-(4-amino-1-oxoisoindolin-2-yl)-1-methylpiperidine-2,6-dione 98%View Details
3-(4-amino-1-oxoisoindolin-2-yl)-1-methylpiperidine-2,6-dione 98%View Details -
 614-19-7 98%View Details
614-19-7 98%View Details
614-19-7 -
 20677-73-0 (2,2-diethoxyethyl)methylamine 98%View Details
20677-73-0 (2,2-diethoxyethyl)methylamine 98%View Details
20677-73-0 -
 3-(4-(hydroxyamino)-1-oxoisoindolin-2-yl)piperidine-2,6-dione 98%View Details
3-(4-(hydroxyamino)-1-oxoisoindolin-2-yl)piperidine-2,6-dione 98%View Details -
 57381-49-4 2-bromo-4-chlorobenzonitrile 98%View Details
57381-49-4 2-bromo-4-chlorobenzonitrile 98%View Details
57381-49-4 -
 4,6-dichloropyrimidine-5-carbaldehyde 98%View Details
4,6-dichloropyrimidine-5-carbaldehyde 98%View Details
5305-40-8
