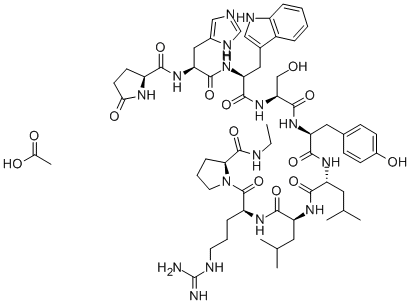
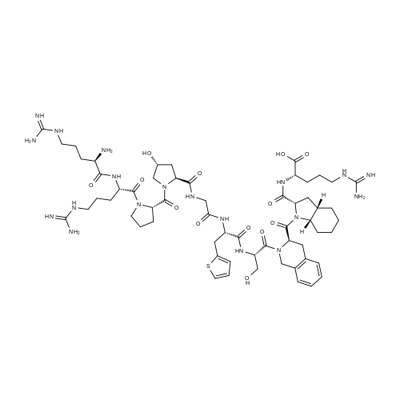
Plecanatide
- CAS NO.:467426-54-6
- Empirical Formula: C65H104N18O26S4
- Molecular Weight: 1681.89
- MDL number: MFCD33029246
- Update Date: 2024-11-18 17:01:59
What is Plecanatide?
Absorption
Plecanatide is minimally absorbed with negligible systemic availability following oral administration. Concentrations of plecanatide and its active metabolite in plasma are below the limit of quantitation after an oral dose of 3 mg. Therefore, standard pharmacokinetic parameters such as AUC, maximum concentration (Cmax), and half-life (t?) cannot be calculated.
Toxicity
Single oral doses of plecanatide at 0.5 mg/kg and 10 mg/kg caused mortality in young juvenile mice on postnatal days 7 and 14, respectively (human age equivalent of approximately 1 month to less than 2 years).
The Uses of Plecanatide
Plecanatide is used in the treatment of chronic idiopathic constipation and irritable bowel syndrome with constipation. It works as a laxative by drawing water in to the gastrointestinal tract thereby softening stool and encouraging its natural passage.
Indications
Plecanatide is indicated for the treatment of chronic idiopathic constipation (CIC) and irritable bowel syndrome with constipation (IBS-C) in adult patients.
Background
Plecanatide is a drug approved in January 2017 by the FDA for the treatment of chronic idiopathic constipation (CIC). It should not be used in children less than six years of age, and should be avoided in patients six years to 18 years of age
Pharmacokinetics
Food Effect Subjects who received either a low-fat, low calorie (LF-LC) meal or a high fat, high calorie (HF-HC) meal reported looser stools than fasted subjects up to 24 hours after a single dose of 9 mg (3 times the recommended dose). In clinical studies, Plecanatide was administered with or without food.
Enzyme inhibitor
This uroguanylin analogue (FW = 1681.90 g/mol; CAS 467426-54-6), also known by the codename SP-304, binds to and activates guanylate cyclase-C (GC-C) receptors (EC50 ≈ 0.3 μM) expressed on the epithelial cells lining turn sequentially activates protein kinase G-II and the chloride channel known as the Cystic Fibrosis Transmembrane-conductance Regulator (CFTR) to regulate ion and fluid transport, to promote epithelial cell homeostasis, and to maintain barrier function in the GI mucosa. Plecanatide is a hexadecapeptide that is structurally identical to uroguanylin, except for an glutamate substitution at position-3. Oral treatment with plecanatide at a dose range between 0.05-2.5 mg/kg per day is as effective as once-daily treatment with 5-amino salicylic acid (100 mg/kg) or sulfasalazine (80 mg/kg). Amelioration of colitis by the treatment with plecanatide or dolcanatide was not dose-dependent, most likely due to saturation of available GC-C receptors.
Metabolism
Plecanatide is metabolized in the GI tract to an active metabolite by loss of the terminal leucine moiety. Both plecanatide and the metabolite are proteolytically degraded within the intestinal lumen to smaller peptides and naturally occurring amino acids.
Properties of Plecanatide
| Boiling point: | 2120.0±65.0 °C(Predicted) |
| Density | 1.47±0.1 g/cm3(Predicted) |
| pka | 3.61±0.21(Predicted) |
| form | Solid |
| color | White to off-white |
Safety information for Plecanatide
Computed Descriptors for Plecanatide
| InChIKey | NSPHQWLKCGGCQR-DLJDZFDSSA-N |
Abamectin manufacturer
Anthem Biosciences Pvt Ltd
New Products
4-AMINO-TETRAHYDRO-PYRAN-4-CARBOXYLIC ACID HCL 4-(Dimethylamino)tetrahydro-2H-pyran-4-carbonitrile 4-Aminotetrahydropyran-4-carbonitrile Hydrochloride (R)-3-Aminobutanenitrile Hydrochloride 3-((Dimethylamino)methyl)-5-methylhexan-2-one oxalate 1,4-Dioxa-8-azaspiro[4.5]decane 5-Bromo-2-nitropyridine Nimesulide BP Aceclofenac IP/BP/EP Diclofenac Sodium IP/BP/EP/USP Mefenamic Acid IP/BP/EP/USP Ornidazole IP Diclofenac Potassium THOMAIND PAPER PH 2.0 TO 4.5 1 BOX BUFFER CAPSULE PH 9.2 - 10 CAP SODIUM CHLORIDE 0.1N CVS ALLOXAN MONOHYDRATE 98% PLATINUM 0.5% ON 3 MM ALUMINA PELLETS (TYPE 73) LITHIUM AAS SOLUTION 2-Bromo-1-(bromomethyl)-3-chloro-5-nitrobenzene 2-Bromo-3-nitroaniline N-(3-Hydroxypropyl)-N-methylacetamide 3-Bromo-6-chloropyridazine 4-ethyl-3-nitrobenzoic acidRelated products of tetrahydrofuran
You may like
-
 467426-54-6 Plecanatide 99%View Details
467426-54-6 Plecanatide 99%View Details
467426-54-6 -
 1-Methyl-6-oxo-1,6-dihydropyridazine-3-carbonitrile 98%View Details
1-Methyl-6-oxo-1,6-dihydropyridazine-3-carbonitrile 98%View Details
99903-60-3 -
 1823368-42-8 98%View Details
1823368-42-8 98%View Details
1823368-42-8 -
 2-(3-(tert-butyl)phenoxy)-2-methylpropanoic acid 1307449-08-6 98%View Details
2-(3-(tert-butyl)phenoxy)-2-methylpropanoic acid 1307449-08-6 98%View Details
1307449-08-6 -
 Ethyl 3-(furan-2-yl)-3-hydroxypropanoate 25408-95-1 98%View Details
Ethyl 3-(furan-2-yl)-3-hydroxypropanoate 25408-95-1 98%View Details
25408-95-1 -
 2-Chloro-5-fluoro-1-methoxy-3-methylbenzene 98%View Details
2-Chloro-5-fluoro-1-methoxy-3-methylbenzene 98%View Details
1805639-70-6 -
 1784294-80-9 98%View Details
1784294-80-9 98%View Details
1784294-80-9 -
 Lithium ClavulanateView Details
Lithium ClavulanateView Details
61177-44-4