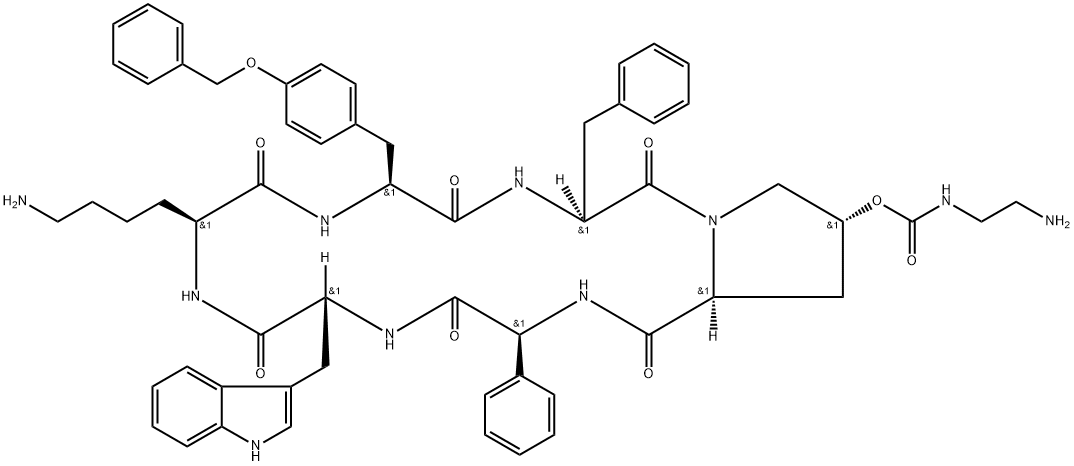
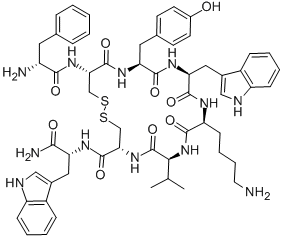
Sincalide
Synonym(s):CCK-8;Cholecystokinin octapeptide;Pancreozymin C-terminal octapeptide;Sincalide
- CAS NO.:25126-32-3
- Empirical Formula: C49H62N10O16S3
- Molecular Weight: 1143.27
- MDL number: MFCD00079849
- EINECS: 246-639-0
- SAFETY DATA SHEET (SDS)
- Update Date: 2024-11-14 14:09:35
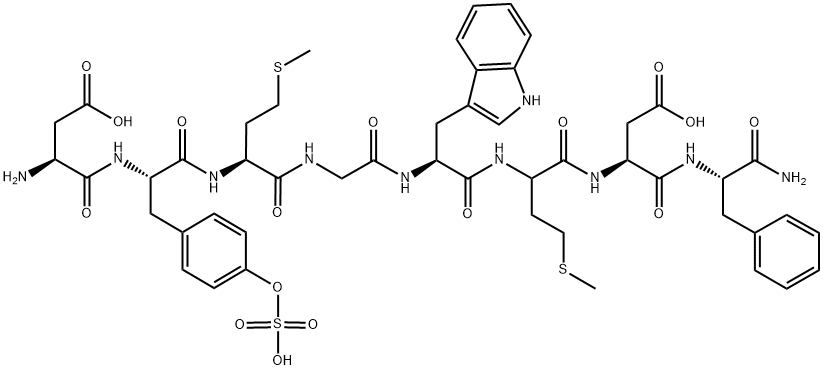
What is Sincalide?
Originator
Kinevac,Squibb,US,1976
The Uses of Sincalide
Sincalide is used in the biological studies in the preparation of opioid receptors agonistic and CCK receptor antagonistic activity.
The Uses of Sincalide
Choleretic.
Definition
ChEBI: Sincalide is an oligopeptide.
Manufacturing Process
The starting material in the following synthesis is: t-butyloxycarbonyl-Laspartyl-
L-tyrosyl-L-methionylglycyl-L-tryptophyl-L-methionyl-L-aspartyl-Lphenylalanine
amide designated (SM)
.(A) A solution of (SM) (320 mg) in trifluoroacetic acid (7 ml) was kept under
nitrogen at room temperature for 15 minutes. Ether (100 ml) was added and
the precipitate filtered, washed thoroughly with ether and dried. This material
(280 mg) was added to concentrated sulfuric acid (20 ml), cooled at -20°C.
The solution was kept in the dry ice-acetone bath at -20°C for 75 minutes.
The sulfuric acid solution was poured into ice water (80 ml). The precipitate
was centrifuged, resuspended in ice water (30 ml) and 4N sodium hydroxide
was added until a clear solution was obtained. After reacidification to pH 4
with dilute sulfuric acid, the precipitate formed was centrifuged, washed twice
with ice water and dried. Yield 155 mg. Chromatograph of DEAE Sephadex
(with ammonium carbonate buffer) yielded the desired octapeptide sulfate
ester: 30 mg.
(B) A solution of (SM) (330 mg) in trifluoroacetic acid (7 ml) was kept under
nitrogen at room temperature for 15 minutes. Ether (100 ml) was added and
the precipitate was filtered, washed thoroughly with ether and dried. This
material (300 mg) was added in portions to concentrated sulfuric acid (18 ml)
cooled at -20°C with vigorous stirring. After 15 minutes a solution of
potassium bisulfate in concentrated sulfuric acid (408 mg in 3 ml) was added.
The reaction mixture was stirred for 75 minutes at -15°C and then stored at -
7°C for 285 minutes. The sulfuric acid solution was poured into cold ether
(400 ml); precipitate was filtered, washed with cold ether, and suspended in
cold water. Complete solution was then achieved by careful addition of 2N
sodium hydroxide. Acidification with N hydrochloric acid led to the
precipitation of the desired octapeptide sulfate ester. Yield 200 mg.
brand name
Kinevac (Bracco).
Therapeutic Function
Choleretic
General Description
It was thought originally that cholecystokinin and pancreozyminwere two different hormones. Cholecystokinin wasthought to be responsible for contraction of the gallbladder,whereas pancreozymin was believed to induce secretion ofpancreatic enzymes. It is now clear that both actions arecaused by a single 33-residue polypeptide, referred to ascholecystokinin–pancreozymin (CCK-PZ). CCK-PZ is secretedin the blood in response to the presence of food in theduodenum, especially long-chain fatty acids. The fiveCOOH-terminal amino acid residues are identical with thosein gastrin. The COOH-terminal octapeptide retains full activityof the parent hormone.
Biochem/physiol Actions
Neurotransmitter; predominant form of CCK in CNS and gastrointestinal tract; may play a role in satiety.
storage
Store at -20°C
Properties of Sincalide
| Melting point: | >193°C (dec.) |
| alpha | D23 -18.4° (c = 0.7 in 1N NH3) |
| Density | 1.440±0.06 g/cm3(Predicted) |
| storage temp. | -20°C |
| solubility | 0.05 M ammonium hydroxide: 1 mg/mL, clear, colorless |
| form | powder |
| pka | -4.17±0.18(Predicted) |
| color | Off-White |
| Water Solubility | Soluble in water 1 g/L at 10°C. |
| Merck | 13,8617 |
| BRN | 5231801 |
Safety information for Sincalide
| Signal word | Warning |
| Pictogram(s) |
 Exclamation Mark Irritant GHS07 |
| GHS Hazard Statements |
H315:Skin corrosion/irritation H319:Serious eye damage/eye irritation H335:Specific target organ toxicity, single exposure;Respiratory tract irritation |
| Precautionary Statement Codes |
P260:Do not breathe dust/fume/gas/mist/vapours/spray. P271:Use only outdoors or in a well-ventilated area. P280:Wear protective gloves/protective clothing/eye protection/face protection. |
Computed Descriptors for Sincalide
| InChIKey | IZTQOLKUZKXIRV-IVVJSLGPSA-N |
Sincalide manufacturer
New Products
Tert-butyl bis(2-chloroethyl)carbamate 4-Methylphenylacetic acid N-Boc-D-alaninol N-BOC-D/L-ALANINOL N-octanoyl benzotriazole 3-Morpholino-1-(4-nitrophenyl)-5,6-dihydropyridin- 2(1H)-one Furan-2,5-Dicarboxylic Acid DIETHYL AMINOMALONATE HYDROCHLORIDE 1,1’-CARBONYLDIIMIDAZOLE R-2-BENZYLOXY PROPIONIC ACID 1,1’-CARBONYLDI (1,2-4 TRIAZOLE) N-METHYL INDAZOLE-3-CARBOXYLIC ACID (2-Hydroxyphenyl)acetonitrile 4-Bromopyrazole 5-BROMO-2CYANO PYRIDINE 5,6-Dimethoxyindanone 5-broMo-2-chloro-N-cyclopentylpyriMidin-4-aMine 2-(Cyanocyclohexyl)acetic acid 4-methoxy-3,5-dinitropyridine 1-(4-(aminomethyl)benzyl)urea hydrochloride 2-aminopropyl benzoate hydrochloride diethyl 2-(2-((tertbutoxycarbonyl)amino) ethyl)malonate tert-butyl 4- (ureidomethyl)benzylcarbamate Ethyl-2-chloro((4-methoxyphenyl)hydrazono)acetateRelated products of tetrahydrofuran
You may like
-
 25126-32-3 98%View Details
25126-32-3 98%View Details
25126-32-3 -
![(Tyr[SO3H]27)Cholecystokinin fragment 26-33 Amide CAS 25126-32-3](https://img.chemicalbook.in//Content/image/CP5.jpg) (Tyr[SO3H]27)Cholecystokinin fragment 26-33 Amide CAS 25126-32-3View Details
(Tyr[SO3H]27)Cholecystokinin fragment 26-33 Amide CAS 25126-32-3View Details
25126-32-3 -
 1975-50-4 98%View Details
1975-50-4 98%View Details
1975-50-4 -
 2-HYDROXY BENZYL ALCOHOL 98%View Details
2-HYDROXY BENZYL ALCOHOL 98%View Details
90-01-7 -
 2-Chloro-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate 221615-75-4 98%View Details
2-Chloro-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate 221615-75-4 98%View Details
221615-75-4 -
 14714-50-2 (2-Hydroxyphenyl)acetonitrile 98+View Details
14714-50-2 (2-Hydroxyphenyl)acetonitrile 98+View Details
14714-50-2 -
 118753-70-1 98+View Details
118753-70-1 98+View Details
118753-70-1 -
 733039-20-8 5-broMo-2-chloro-N-cyclopentylpyriMidin-4-aMine 98+View Details
733039-20-8 5-broMo-2-chloro-N-cyclopentylpyriMidin-4-aMine 98+View Details
733039-20-8