PIPES
Synonym(s):1,4-Piperazinediethanesulfonic acid;Piperazine-1,4-bis(2-ethanesulfonic acid);Piperazine-N,N′-bis(2-ethanesulfonic acid);PIPES
- CAS NO.:5625-37-6
- Empirical Formula: C8H18N2O6S2
- Molecular Weight: 302.37
- MDL number: MFCD00006159
- EINECS: 227-057-6
- SAFETY DATA SHEET (SDS)
- Update Date: 2024-12-18 14:08:52
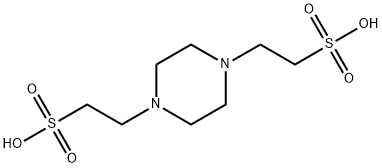
What is PIPES?
Description
PIPES is a member of the ethanesulfonic acid buffer series, first introduced by Good et al., developed to meet certain criteria: midrange pKa, maximum water solubility and minimum solubility in all other solvents, minimal salt effects, minimal change in pKa with temperature, chemically and enzymatically stable, minimal absorption in visible or UV spectral range and easily synthesized. Since its pKa at 37 °C is near physiological pH, PIPES has applications in cell culture work.
Chemical properties
White/clear crystalline powder
The Uses of PIPES
Protocols have been reported on the use of PIPES for separation of glyoxylated RNA in agarose gels, nuclease S1 mapping of RNA, and in ribonuclease protection assay protocols. PIPES has been used as a buffer in glutaraldehyde fixation of tissue samples.,
PIPES has been utilized in protein crystallization., The use of PIPES in the reconstitution of dissociated tubulin
α and β subunits after their resolution on immunoadsorbent gels has been described. PIPES has been recommended for use in buffers for the in vitro study of caspases 3, 6, 7, and 8.
A published study demonstrated the usefulness of PIPES as a non-metal ion complexing buffer in such applications as protein assays. PIPES has been used in cell culture for such applications as the engineering of a thermostable mutant membrane protein in Escherichia coli.
The Uses of PIPES
A buffering agent with a pKa near physiological pH.PIPES [piperazine-N,N′-bis(2-ethanesulfonic acid)] is frequently used as a buffering agent in biochemistry. It is an ethanesulfonic acid buffer developed by Good et al. in the 1960s. PIPES has a pKa near the physiological pH which makes it useful in cell culture work. It has been documented to minimize lipid loss when buffering glutaraldehyde histology in plant and animal tissues.
Additional forms available: PIPES, Sesquisodium Salt ; PIPES dipotassium salt; PIPES disodium salt; PIPES, Sodium Salt. Buffers can be prepared by adding a solution of base to PIPES free acid, titrating to the appropriate pH, or by mixing equimolar solutions of the monosodium salt and the disodium salt, titrating to the appropriate pH.
Definition
ChEBI: 2,2'-piperazine-1,4-diylbisethanesulfonic acid is a Good's buffer substance, pKa = 6.8 at 20 ℃. It is a conjugate acid of a 2,2'-piperazine-1,4-diylbisethanesulfonate.
What are the applications of Application
Glutaraldehyde fixation of plant and animal tissue samples can cause loss of lipid, leading to apparent morphological changes. Lipid loss and artifacts were minimized when PIPES was used to buffer the glutaraldehyde fixative.
Alkaline phosphatase activity is lost selectively from certain rat hepatocyte organelles when fixed for ultracytochemistry with cacodylate-buffered glutaraldehyde. When PIPES was used as buffer, retention of activity was 60% greater.
Fixation of fungal zoospores for fluorescence microscopy and electron microscopy was optimal with a combination of glutaraldehyde and formaldehyde in PIPES buffer.
What are the applications of Application
PIPES, Free Acid is a buffering agent with a pKa near physiological pH
General Description
PIPES is a member of the ethanesulfonic acid buffer series, first introduced by Good et al., developed to meet certain criteria: midrange pKa, maximum water solubility and minimum solubility in all other solvents, minimal salt effects, minimal change in pKa with temperature, chemically and enzymatically stable, minimal absorption in visible or UV spectral range and easily synthesized. Since its pKa at 37 °C is near physiological pH, PIPES has applications in cell culture work.
Flammability and Explosibility
Not classified
Purification Methods
Purify PIPES from boiling water (maximum solubility is about 1g/L) or as described for ADA [N-(2-acetamido)iminodiacetic acid above]. [Good et al. Biochemistry 5 469 1966, Beilstein 23/12 V 380.]
Properties of PIPES
| Melting point: | >300 °C (lit.) |
| Density | 1.4983 (rough estimate) |
| refractive index | 1.6300 (estimate) |
| storage temp. | Sealed in dry,Room Temperature |
| solubility | 0.1 M NaOH: 0.25 g/mL, clear, colorless |
| form | Micro-Crystalline Powder |
| color | White |
| PH Range | 6.1 - 7.5 |
| pka | 6.8(at 25℃) |
| Water Solubility | Soluble in water. |
| λmax | λ: 260 nm Amax: 0.055 λ: 280 nm Amax: 0.040 |
| Merck | 14,7479 |
| BRN | 817713 |
| CAS DataBase Reference | 5625-37-6(CAS DataBase Reference) |
| EPA Substance Registry System | 1,4-Piperazinediethanesulfonic acid (5625-37-6) |
Safety information for PIPES
| Signal word | Warning |
| Pictogram(s) |
 Exclamation Mark Irritant GHS07 |
| GHS Hazard Statements |
H315:Skin corrosion/irritation H319:Serious eye damage/eye irritation H335:Specific target organ toxicity, single exposure;Respiratory tract irritation |
| Precautionary Statement Codes |
P261:Avoid breathing dust/fume/gas/mist/vapours/spray. P271:Use only outdoors or in a well-ventilated area. P280:Wear protective gloves/protective clothing/eye protection/face protection. |
Computed Descriptors for PIPES
| InChIKey | XWGRYGKFRRLUQQ-UHFFFAOYSA-N |
PIPES manufacturer
ARRAKIS INDUSTRIES LLP
Anand Agencies
New Products
(S)-3-Aminobutanenitrile hydrochloride 4-Methylphenylacetic acid N-Boc-D-alaninol N-BOC-D/L-ALANINOL Tert-butyl bis(2-chloroethyl)carbamate 3-Morpholino-1-(4-nitrophenyl)-5,6-dihydropyridin- 2(1H)-one Furan-2,5-Dicarboxylic Acid Tropic acid 1-Bromo-3,5-Di-Tert-Butylbenzene S-2-CHLORO PROPIONIC ACID ETHYL ISOCYANOACETATE 2-Bromo-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate 4-IODO BENZOIC ACID 3-NITRO-2-METHYL ANILINE 1-(2,4-DICHLOROPHENYL) ETHANAMINE (2-Hydroxyphenyl)acetonitrile 4-Bromopyrazole 2-(Cyanocyclohexyl)acetic acid 4-methoxy-3,5-dinitropyridine 1-(4-(aminomethyl)benzyl)urea hydrochloride 2-aminopropyl benzoate hydrochloride diethyl 2-(2-((tertbutoxycarbonyl)amino) ethyl)malonate tert-butyl 4- (ureidomethyl)benzylcarbamate Ethyl-2-chloro((4-methoxyphenyl)hydrazono)acetateRelated products of tetrahydrofuran



You may like
-
 PIPES Buffer for molecular biology CAS 5625-37-6View Details
PIPES Buffer for molecular biology CAS 5625-37-6View Details
5625-37-6 -
 PIPES Buffer for tissue culture CAS 5625-37-6View Details
PIPES Buffer for tissue culture CAS 5625-37-6View Details
5625-37-6 -
 PIPES buffer soln. CAS 5625-37-6View Details
PIPES buffer soln. CAS 5625-37-6View Details
5625-37-6 -
 PIPES Buffer extrapure CAS 5625-37-6View Details
PIPES Buffer extrapure CAS 5625-37-6View Details
5625-37-6 -
 PIPES CAS 5625-37-6View Details
PIPES CAS 5625-37-6View Details
5625-37-6 -
 Pipes, buffer CAS 5625-37-6View Details
Pipes, buffer CAS 5625-37-6View Details
5625-37-6 -
 PIPES 99% CAS 5625-37-6View Details
PIPES 99% CAS 5625-37-6View Details
5625-37-6 -
 PIPES BUFFER CAS 5625-37-6View Details
PIPES BUFFER CAS 5625-37-6View Details
5625-37-6