Dihydroergotoxine mesylate
- CAS NO.:8067-24-1
- Empirical Formula: C20H29N3O5S
- Molecular Weight: 423.53
- MDL number: MFCD00209881
- SAFETY DATA SHEET (SDS)
- Update Date: 2024-08-07 19:09:42
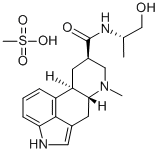
What is Dihydroergotoxine mesylate?
Absorption
The maximal plasma concentration of the components of the mixture of ergoloid mesylates is approximately 0.04 mcg/l. The analysis of the concentration of the major metabolites of each component indicates that all ergoloid mesylate are rapidly transformed after absorption. This finding is supported as they were found in one order of magnitude higher in concentration when compared to the original form. Ergoloid mesylate seems to have a very low absorption of only 25%. From this absorption by the GI tract, approximately 50% of the absorbed dose is eliminated by first-pass metabolism. After absorption, the maximal plasma concentration is attained after 3-4 hours and the oral bioavailability is very low. Simultaneous food ingestion has no effect on the extent of absorption but it does lower the absorption rate. To know more about the individual components of the ergoloid mixture, please visit Dihydro-alpha-ergocryptine, Dihydroergocornine and Dihydroergocristine.
Toxicity
Symptoms of overdose include dyspnea, hypotension or hypertension, rapid weak pulse, delirium, nausea, vomiting, and bradycardia. The excessive use of ergoloid mesylate can lead to ergotism. To know more about the individual components of the ergoloid mixture, please visit Dihydro-alpha-ergocryptine, Dihydroergocornine and Dihydroergocristine.
Description
Ergoloid is a mixture of the ergot alkaloids dihydroergocornine, dihydroergocrystine, and dihydroergocryptine. It blocks reflex vasodilation and vasoconstriction induced by norepinephrine in dogs when injected into the kidney at a dose of 0.15 mg. Ergoloid blocks norepinephrine-induced cAMP production in rat brain cortical homogenates ex vivo in a dose-dependent manner. It also acts as an erectogenic agent in rats. Formulations containing ergoloid have been used in the treatment of migraine headaches and vascular dementias.
The Uses of Dihydroergotoxine mesylate
Cognition adjuvant.
Indications
It was labeled by the FDA for the treatment of symptoms of an idiopathic decline in the mental capacity not related to a potentially reversible condition as well as for age-related cognitive impairment. The prescription of this drug is conditioned to the corroboration that the patient is not suffering from a potentially reversible and treatable condition especially delirium and dementiform illness secondary to systemic disease, primary neurological disease or primary mood disturbance. To know more about the individual components of the ergoloid mixture, please visit Dihydroergocristine and Dihydro-alpha-ergocryptine
Background
Ergoloid Mesylate is an equiproportional preparation of three different ergotamantriones: dihydroergocornine, dihydroergocristine, and dihydroergocryptine. All these components are produced by the fungus Claviceps purpurea and are all derivatives of the tetracyclic compound 6-methylergonovine. The derivatives of this fungus are identified to be about 350 different substances from which the components of the ergoloid mesylate mixture are composed of the dihydrogenated ergot alkaloid derivatives. The mixture of ergoloid mesylate was first developed by Novartis and FDA approved on November 5, 1953, but this specific formulation is now discontinued.Later in 1991, the mixture of ergoloid mesylates was retaken by Sun Pharmaceutical Industries and approved by the FDA. To know more about the individual components of the ergoloid mixture, please visit Epicriptine, Dihydro-alpha-ergocryptine, Dihydroergocornine and Dihydroergocristine.
What are the applications of Application
Dihydroergotoxine mesylate is binds with high affinity to GABAA receptor
Definition
ChEBI: Dihydroergotoxine mesylate is a mixture of hydrogenated ergot alkaloids consisting of the methanesulfonic acid salts of dihydroergocornine (R = CHMe2), dihydroergocristine (R = CH2Ph), and alpha- and beta-dihydroer ocryptine (R = CH2CHMe2 and CH(Me)Et, respectively). It is used for the symptomatic treatment of mild to moderate dementia in the elderly, although its value is not established.
brand name
Alkergot (Sandoz); Circanol(3M Pharmaceuticals); Deapril (Bristol-Myers Squibb);Gerimal (Watson); Hydergine (Novartis).
Biological Activity
Dihydroergotoxine mesylate is a complex of closely related alkaloid salts. Binds with high affinity to the GABAA receptor Cl- channel, producing an allosteric interaction with the benzodiazepine site. Also interacts with central dopaminergic, serotonergic and adrenergic (α1) receptors. Displays antiproliferative activity in vitro (IC50 = 18 - 38 μM in prostate cancer cells) and exhibits cognition-enhancing, anticonvulsant and sedative activity in vivo.
Pharmacokinetics
The mechanism of action of ergoloid mesylate is not completely established but it is thought that it increases both the blood flow and cerebral metabolism. Some studies have shown a significant improvement in alertness and memory, as well as a decline in confusion while some other benefits have shown a lack of effect. Its action produces a damper in the impulses from the vasomotor center which reduces the vascular tone which translates in the slow of pulse rate, prevention of compensatory tachycardia. To know more about the individual components of the ergoloid mixture, please visit Dihydro-alpha-ergocryptine, Dihydroergocornine and Dihydroergocristine.
Pharmacology
The metabolic effects of ergoloid mesylates (Hydergine) may include enhancement of noradrenergic, dopaminergic and serotoninergic neurotransmission (Weil,1988) and reduction of free radicals. Schneider and Olin (1994) reviewed 151 reports on the use of Hydergine in senile dementia, but only 47 of these trials (31%) met strict criteria for meta-analysis. ACochrane review (Olin et al., 2001) confirmed that patients with VaD appeared to benefit more than patients with AD in terms of cognition,clinical global ratings and combined measures. However, compared with placebo, efficacy was very modest. Thus, there are no grounds to recommend its use.
Metabolism
Most of the metabolism of ergoloid mesylate is hepatic and it is performed very rapidly after absorption. After exposition in vitro, the major metabolites identified are hydroxy-dihydroergocornine, hydroxy-dihydroergocryptine and hydroxy-dihydroergocristine. All metabolic transformations seem to be related to the activity of the cytochrome CYP3A4. To know more about the individual components of the ergoloid mixture, please visit Dihydro-alpha-ergocryptine, Dihydroergocornine and Dihydroergocristine.
Properties of Dihydroergotoxine mesylate
| storage temp. | Store at RT |
| solubility | DMSO: Slightly Soluble; Methanol: 1 mg/ml |
| form | neat |
| form | Solid |
| color | White to off-white |
Safety information for Dihydroergotoxine mesylate
| Signal word | Warning |
| Pictogram(s) |
 Exclamation Mark Irritant GHS07 |
| GHS Hazard Statements |
H302:Acute toxicity,oral |
Computed Descriptors for Dihydroergotoxine mesylate
| InChIKey | ONYMVHQTXOEAOV-TZWRWDCZNA-N |
| SMILES | C12C3=CC=CC=1[C@@]1([H])C[C@H](CN(C)[C@]1([H])CC2=CN3)C(=O)N[C@@H](C)CO.S(=O)(=O)(O)C |&1:6,9,13,22,r| |
Related products of tetrahydrofuran


You may like
-
 Ergoloid mesylates CAS 8067-24-1View Details
Ergoloid mesylates CAS 8067-24-1View Details
8067-24-1 -
 37951-47-6 3'-Benzyloxy propiophenone, 98% 99%View Details
37951-47-6 3'-Benzyloxy propiophenone, 98% 99%View Details
37951-47-6 -
 99-30-9 99%View Details
99-30-9 99%View Details
99-30-9 -
 104944-18-5 99%View Details
104944-18-5 99%View Details
104944-18-5 -
 3'-Methoxypropiophenone, 99% 37951-49-8 99%View Details
3'-Methoxypropiophenone, 99% 37951-49-8 99%View Details
37951-49-8 -
 98-56-6 99%View Details
98-56-6 99%View Details
98-56-6 -
 694-48-4 99%View Details
694-48-4 99%View Details
694-48-4 -
 51364-51-3 99%View Details
51364-51-3 99%View Details
51364-51-3