methanesulfonate
- CAS NO.:138540-32-6
- Empirical Formula: C22H29N5O5S
- Molecular Weight: 475.56116
- SAFETY DATA SHEET (SDS)
- Update Date: 2022-12-21 16:56:50
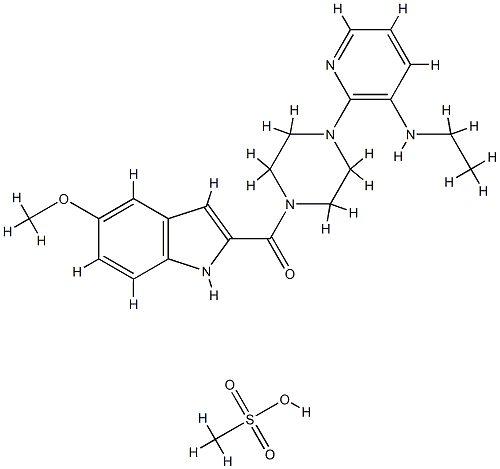
What is methanesulfonate?
Originator
Atevirdine,Upjohn
Manufacturing Process
1-[5-Methoxyindolyl-2-carbonyl]-4-[3-(ethylamino)-2-pyridinyl]piperazine:
1,1'-Carbonyldiimidazole (0.55 g) is added to a 20°-25°C solution of 5-
methoxyindole-2-carboxylic acid (0.59 g) in tetrahydrofuran (7.0 ml). After
stirring 1 hour, the reaction is transferred via cannnula into a solution of 1-(3-
N-ethylamino-2-pyridinyl)piperazine (0.70 g) in tetrahydrofuran (7 ml) at -
12°C (ice/acetone bath). The reaction is stirred at -10°C for 30 minutes, then
slowly warmed to 20°-25°C and stirred a further 18 hours. After diluting with ether (60 ml), the mixture is washed with saturated aqueous sodium bicarbonate (70 ml), saline (70 ml) and dried over anhydrous sodium sulfate.
The mixture is concentrated under reduced pressure to a residue which is
purified by flash chromatography (2 cm x 20 cm) eluting with
methanol/chloroform (2/98) to give the title compound, mp 153°-154°C.
1-[5-Methoxyindolyl-2-carbonyl]-4-[3-(ethylamino)-2-pyridinyl]piperazine
hydrochloride:
1-(Ethyl)-3-(dimethylaminopropyl)carbodiimide (1.25 g) is added to a solution
of 1-(3-ethyl-2-pyridinyl)piperazine (1.12 g) in THF (15 ml). The reaction is
stirred at 20°-25°C for 3 hr, then it is dissolved in chloroform (50 ml) and
extracted with saturated aqueous sodium bicarbonate, saline, dried over
anhydrous sodium sulfate and concentrated under reduced pressure.
Purification by flash column chromatography (200 g silica) eluting with ethyl
acetate/hexane (50/50), the appropriate fractions are pooled and
concentrated to give the title compound, The product is dissolved in methanol
(150 ml) with heating, cooled to 20°-25°C and chlorotrimethylsilane (4.70
mmol) is added. The mixture is concentrated to half-volume, ether is added
until cloudy and the flask is stored at 0°C overnight. Filtration gives the
hydrochloride salt, MP: 194°-195°C.
The mesylate salt is formed by dissolving the free base in methanol and by
adding methanesulfonic acid (1 eq). The solution is diluted with diethyl ether
until the salt crystallizes out of solution. The crystals are collected and dried
to afford the mesyl salt of the title compound, MP: 215°-216°C.
Therapeutic Function
Antiviral
Properties of methanesulfonate
| Melting point: | 215-216° |
Safety information for methanesulfonate
Computed Descriptors for methanesulfonate
New Products
(S)-3-Aminobutanenitrile hydrochloride 4-Methylphenylacetic acid N-Boc-D-alaninol N-BOC-D/L-ALANINOL Tert-butyl bis(2-chloroethyl)carbamate 3-Morpholino-1-(4-nitrophenyl)-5,6-dihydropyridin- 2(1H)-one Furan-2,5-Dicarboxylic Acid Tropic acid 1-Bromo-3,5-Di-Tert-Butylbenzene S-2-CHLORO PROPIONIC ACID ETHYL ISOCYANOACETATE 2-Bromo-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate 4-IODO BENZOIC ACID 3-NITRO-2-METHYL ANILINE 1-(2,4-DICHLOROPHENYL) ETHANAMINE (2-Hydroxyphenyl)acetonitrile 4-Bromopyrazole 2-(Cyanocyclohexyl)acetic acid 4-methoxy-3,5-dinitropyridine 1-(4-(aminomethyl)benzyl)urea hydrochloride 2-aminopropyl benzoate hydrochloride diethyl 2-(2-((tertbutoxycarbonyl)amino) ethyl)malonate tert-butyl 4- (ureidomethyl)benzylcarbamate Ethyl-2-chloro((4-methoxyphenyl)hydrazono)acetateYou may like
-
 2033-24-1 98%View Details
2033-24-1 98%View Details
2033-24-1 -
 42831-50-5 5-METHYLISOXAZOLE-4-CARBOXYLIC ACID 98%View Details
42831-50-5 5-METHYLISOXAZOLE-4-CARBOXYLIC ACID 98%View Details
42831-50-5 -
 1975-50-4 98%View Details
1975-50-4 98%View Details
1975-50-4 -
 2-HYDROXY BENZYL ALCOHOL 98%View Details
2-HYDROXY BENZYL ALCOHOL 98%View Details
90-01-7 -
 2-Chloro-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate 221615-75-4 98%View Details
2-Chloro-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate 221615-75-4 98%View Details
221615-75-4 -
 61397-56-6 CIS BROMO BENZOATE 98%View Details
61397-56-6 CIS BROMO BENZOATE 98%View Details
61397-56-6 -
 14714-50-2 (2-Hydroxyphenyl)acetonitrile 98+View Details
14714-50-2 (2-Hydroxyphenyl)acetonitrile 98+View Details
14714-50-2 -
 118753-70-1 98+View Details
118753-70-1 98+View Details
118753-70-1