Itraconazole
Synonym(s):;Itraconazole;Oriconazole;Sporanox
- CAS NO.:84625-61-6
- Empirical Formula: C35H38Cl2N8O4
- Molecular Weight: 705.63
- MDL number: MFCD00870168
- EINECS: 617-596-9
- SAFETY DATA SHEET (SDS)
- Update Date: 2025-12-23 13:58:55
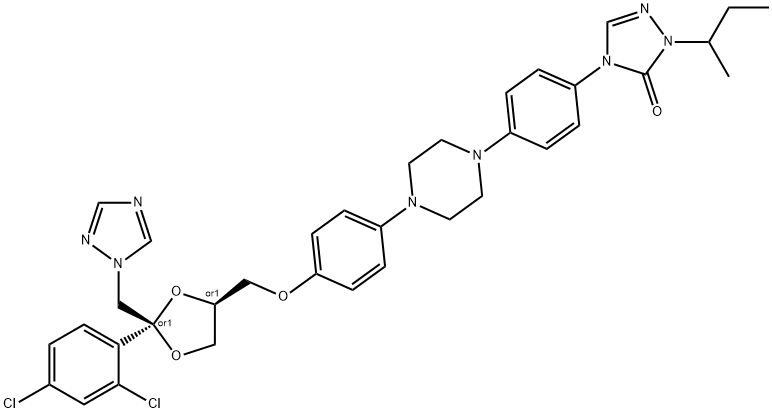
What is Itraconazole?
Absorption
The absolute oral bioavailability of itraconazole is 55%, and is maximal when taken with a full meal.
Toxicity
No significant lethality was observed when itraconazole was administered orally to mice and rats at dosage levels of 320 mg/kg or to dogs at 200 mg/kg.
Description
Itraconazole is an orally-active triazole antifungal indicated for use in the treatment of dermal, vaginal and systemic mycoses. In immunocompromised and AIDS patients, itraconazole has been shown to significantly reduce the incidence of relapses of cryptococcal meningitis.
Chemical properties
Off-White Crystalline Solid
Originator
Janssen (Belgium)
The Uses of Itraconazole
Itraconazole is a triazole antifungal agent. It is used to inhibit cytochrome P-450-dependent enzymes and ergosterol synthesis. It has been used against histoplasmosis, blastomycosis, cryptococcal meningitis, and aspergillosis. It?s different formulations are used to study Candida strains in murine invasive infections. It has been used to study proliferative changes of the forestomach mucosa in alloxan-induced diabetic rats..
The Uses of Itraconazole
An orally active antimycotic structurally related to Ketoconazole. Antifungal
The Uses of Itraconazole
vitamin, enzyme cofactor
The Uses of Itraconazole
Anti-infective
The Uses of Itraconazole
Anti Fungal. Used in the treatment of stomach upset/ indigestion and other gastrointestinal conditions
The Uses of Itraconazole
For the treatment of the following fungal infections in immunocompromised and non-immunocompromised patients: pulmonary and extrapulmonary blastomycosis, histoplasmosis, aspergillosis, and onychomycosis.
The Uses of Itraconazole
A traizole antifungal agent
Background
One of the triazole antifungal agents that inhibits cytochrome P-450-dependent enzymes resulting in impairment of ergosterol synthesis. It has been used against histoplasmosis, blastomycosis, cryptococcal meningitis & aspergillosis.
Indications
For the treatment of the following fungal infections in immunocompromised and non-immunocompromised patients: pulmonary and extrapulmonary blastomycosis, histoplasmosis, aspergillosis, and onychomycosis.
Indications
Itraconazole (Sporanox) is effective in the treatment of histoplasmosis, blastomycosis,
candidiasis, and dermatophyte infection. Its efficacy in the treatment
of tinea capitis in children is equal to griseofulvin, and it is usually better
tolerated (21). It is metabolized by the cytochrome P-450 system and may
increase the levels ofwarfarin, cyclosporine, and digoxin among others. Its use
is contraindicated with certain medications.
Itraconazole (Sporanox) is a triazole antifungal that is related to the imidazole
ketoconazole. Similar to ketoconazole, it interferes with ergosterol
synthesis and cell membrane integrity. It is clinically active against dimorphic
fungi, yeast, dermatophytes, Blastomycetes, histoplasmosis, sporotrichosis,
and Aspergillus.
Itraconazole is a potent inhibitor of the cytochrome P450 3A enzyme
system, which may elevate blood levels of other drugs metabolized by this system
if taken concomitantly. Itraconazole levels may decrease in patients who
are concurrently taking rifampin, phenobarbital, or phenytoin. Cyclosporine,
felodipine, digoxin, warfarin, and oral hypoglycemic levels may increase when
given in conjunction with itraconazole.
Itraconazole, like ketoconazole, is contraindicated in patients taking cisapride.
Itraconazole may induce torsades de pointes, ventricular arrhythmias,
and congestive heart failure.
Definition
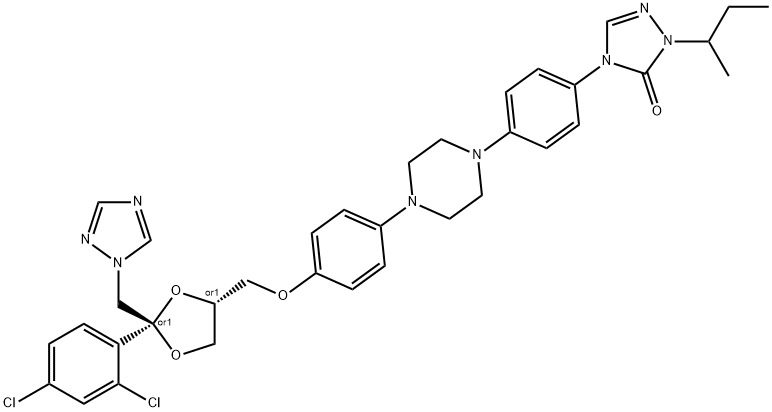
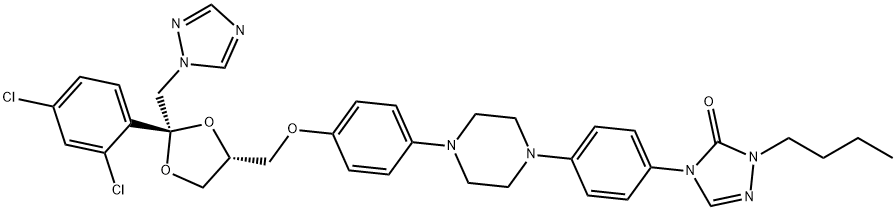
ChEBI: Itraconazole is an N-arylpiperazine that is cis-ketoconazole in which the imidazol-1-yl group is replaced by a 1,2,4-triazol-1-yl group and in which the actyl group attached to the piperazine moiety is replaced by a p-[(+-)1-sec-butyl-5-oxo-1,5-dihydro-4H-1,2,4-triazol-4-yl]phenyl group. A potent P-glycoprotein and CYP3A4 inhibitor, it is used as an antifungal drug for the treatment of various fungal infections, including aspergillosis, blastomycosis, candidiasis, chromoblastomycosis, coccidioidomycosis, cryptococcosis, histoplasmosis, and sporotrichosis. It has a role as a P450 inhibitor, an EC 3.6.3.44 (xenobiotic-transporting ATPase) inhibitor and a Hedgehog signaling pathway inhibitor. It is a member of triazoles, a dioxolane, a N-arylpiperazine, a dichlorobenzene, a cyclic ketal, a conazole antifungal drug, a triazole antifungal drug and an aromatic ether.
Manufacturing Process
Synthesis of cis-4-{4-[4-{4-[2-(2,4-dichlorophenyl)-2-(1H-1,2,4-triazol-1-
ylmethyl)-1,3-dioxolan-4-ylmethoxy]phenyl}-1-piperazinyl]phenyl}-2,4-
dihydro-2-(methylpropyl)-3H-1,2,4-triazol-3-one is showed by the same
procedure as for cis-4-{4-[4-{4-[2-(2,4-dichlorophenyl)-2-(1H-1,2,4-triazol-1-
ylmethyl)-1,3- dioxolan-4-ylmethoxy]phenyl}-1-piperazinyl]phenyl}-2,4-dihydro-2-propyl-3H-1,2,4-triazol-3-one described in the patent.
A mixture of 13.4 parts of 1-(4-methoxyphenyl)piperazine dihydrochloride, 7.9
parts of 1-chloro-4-nitrobenzene, 10 parts of potassium carbonate and 90
parts of N,N-dimethylformamide is stirred and refluxed overnight. The reaction
mixture is diluted with water and the product is extracted twice with
trichloromethane. The residue is triturated in 4-methyl-2-pentanone. The
product is filtered off and crystallized from 1,4-dioxane, yielding 10.5 parts
(67%) of 1-(4-methoxyphenyl)-4-(4-nitrophenyl)piperazine; melting point
195.1°C.
A mixture of 12 parts of 1-(4-methoxyphenyl)-4-(4-nitrophenyl)piperazine,
200 parts of methanol and 225 parts of tetrahydrofuran is hydrogenated at
normal pressure and at 20°C with 2 parts of palladium-on-charcoal catalyst
10%. After the calculated amount of hydrogen is taken up, the catalyst is
filtered off and washed with N,N-dimethylacetamide. Product is filtered off and
crystallized from 1-butanol, yielding 8 parts (74%) of 4-[4-(4-
methoxyphenyl)-1-piperazinyl]benzenamine; melting point 191.8°C.
A mixture of 30 parts of 4-[4-(4-methoxyphenyl)-1-piperazinyl]benzenamine
and 300 parts of a hydrobromic acid solution 48% in water is stirred and
refluxed for 10 days. The reaction mixture is evaporated and the residue is
alkalized with sodium hydroxide. The mixture is filtered and the filtrate is
acidified with acetic acid. The precipitated product is filtered off and
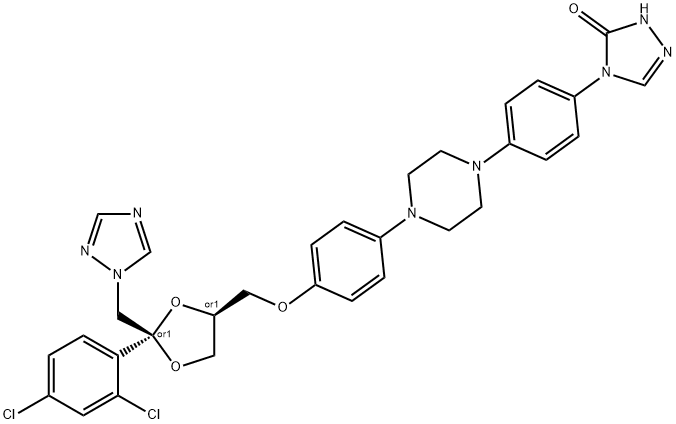
crystallized from 1,4-dioxane, yielding 12 parts (44%) of 2,4-dihydro-4-{4-[4-
(4-hydroxyphenyl)-1-piperazinyl]phenyl}-2-(1-methylpropyl)-3H-1,2,4-triazol-
3-one.
To a stirred solution of 2,4-dihydro-4-{4-[4-(4-hydroxyphenyl)-1-piperazinyl]
phenyl}-2-(1-methylpropyl)-3H-1,2,4-triazol-3-one in 100 parts of dimethyl
sulfoxide are added 0.3 parts of sodium hydride dispersion 78% and the
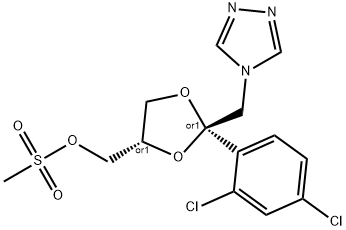
whole is stirred at 50°C till foaming has ceased. Then there are added 3.7
parts of cis-[2-(2,4-dichlorophenyl)-2-(1H-1,2,4-triazol-1-ylmethyl)-1,3-
dioxolan-4-ylmethyl]methanesulfonate and stirring is continued for 3 hours at
100°C. The reaction mixture is cooled and poured onto water. The product is
extracted with dichloromethane. The extracts are washed with a diluted
sodium hydroxide solution and filtered. The residue is crystallized from 1-
butanol. The product yield 4.3 parts (75%) of cis-4-{4-[4-{4-[2-(2,4-
dichlorophenyl)-2-(1H-1,2,4-triazol-1-ylmethyl)-1,3-dioxolan-4-ylmethoxy]
phenyl}-1-piperazinyl]phenyl}-2,4-dihydro-2-(methylpropyl)-3H-1,2,4-triazol-
3-one.
brand name
Sporanox (Janssen).
Therapeutic Function
Antifungal
Antimicrobial activity
The spectrum includes dermatophytes, dimorphic fungi (Blast. dermatitidis, Coccidioides spp., Hist. capsulatum, Paracocc. brasiliensis, Penicillium marneffei and Spor. schenckii), molds (including Aspergillus spp.), dematiaceous fungi and yeasts (Candida spp. and Cryptococcus spp.).
Acquired resistance
This is uncommon, but fluconazole-resistant C. albicans and C. glabrata are often cross-resistant to itraconazole. There are reports of itraconazole-resistant strains of A. fumigatus.
General Description
Itraconazole is an antifungal drug prescribed for oral or intravenous treatment of fungal infections. The drug is sold under trade names such as Sporanoxor Onmel?. This Certified Spiking Solution? is suitable as starting material for calibrators, controls, or linearity standards for clinical and diagnostic testing or therapeutic drug monitoring of itraconazole in patient blood, serum, or plasma samples by LC-MS/MS or HPLC.
Pharmaceutical Applications
A synthetic dioxolane triazole available for oral or parenteral administration.
Biochem/physiol Actions
Itraconazole inhibits cytochrome P-450-dependent enzymes which results in the inhibition of ergosterol synthesis. It does so by interacting with 14-α demethylase, which is a cytochrome P-450 enzyme necessary to convert lanosterol to ergosterol. Ergosterol is a crucial compenent of fungal cell membranes. Therefore, it′s inhibition results in increased cellular permeability causing leakage of cellular contents. Itraconazole may also inhibit endogenous respiration, interact with membrane phospholipids, inhibit the transformation of yeasts to mycelial forms, inhibit purine uptake, and impair triglyceride and phospholipid biosynthesis.
Pharmacokinetics
Itraconazole is an imidazole/triazole type antifungal agent. Itraconazole is a highly selective inhibitor of fungal cytochrome P-450 sterol C-14 α-demethylation via the inhibition of the enzyme cytochrome P450 14α-demethylase. This enzyme converts lanosterol to ergosterol, and is required in fungal cell wall synthesis. The subsequent loss of normal sterols correlates with the accumulation of 14 α-methyl sterols in fungi and may be partly responsible for the fungistatic activity of fluconazole. Mammalian cell demethylation is much less sensitive to fluconazole inhibition. Itraconazole exhibits in vitro activity against Cryptococcus neoformans and Candida spp. Fungistatic activity has also been demonstrated in normal and immunocompromised animal models for systemic and intracranial fungal infections due to Cryptococcus neoformans and for systemic infections due to Candida albicans.
Pharmacokinetics
Oral absorption: 30% (capsules); 55% (solution)
Cmax 100 mg oral: 0.1–0.2 mg/L after 2–4 h
Plasma half-life: 20–30 h
Volume of distribution: 11 L/kg
Plasma protein binding: >99%
Absorption
Absorption is improved if the drug is given with food or an acidic beverage. In contrast, absorption is reduced if it is given together with compounds that reduce gastric acid secretion. Higher concentrations are obtained with repeated dosing, but there is much individual variation. Incorporation into a solution of hydroxypropyl-β-cyclodextrin enhances bioavailability and leads to much higher blood levels in neutropenic individuals and persons with AIDS. This formulation is better absorbed if given without food. Increases in dosage produce disproportionate changes in blood concentrations.
Distribution
Levels in the CSF are low, but concentrations in lung, liver and bone are 2–3 times higher than in serum, and concentrations in the genital tract are 3–10 times higher. High concentrations are also found in the stratum corneum, as a result of drug secretion in sebum. The drug persists in the skin and nails for weeks to months after treatment is discontinued.
Metabolism and excretion
It is degraded by the liver into a large number of (mostly inactive) metabolites which are excreted with the bile and urine. Itraconazole is unusual because the major metabolite, hydroxyitraconazole, is bioactive and has a similar spectrum of activity as the parent compound. In the steady state, this metabolite is found at serum concentrations about two-fold higher than those of the parent drug. About 80–90% of the intravenous carrier, hydroxypropyl-β-cyclodextrin, is excreted unchanged in the urine. No adjustment of dosage is required in hepatic or renal failure, or during hemodialysis or peritoneal dialysis.
Clinical Use
Aspergillosis
Systemic mycoses with dimorphic fungi (blastomycosis,
coccidioidomycosis, histoplasmosis, paracoccidioidomycosis, penicilliosis)
Subcutaneous mycoses (chromoblastomycosis, sporotrichosis)
Mucosal and cutaneous candidosis.
Dermatophytosis
Phaeohyphomycosis
Pityriasis versicolor
Side Effects
Unwanted effects are more common with oral solution than
with capsules, and are more severe. They include nausea,
abdominal discomfort, dyspepsia, diarrhea, headache, pruritus
and skin rash. Rare side effects include Stevens–Johnson
syndrome, transient abnormalities of liver enzymes, reversible
idiosyncratic hepatitis and hypokalemia.
Intravenous itraconazole has been associated with congestive
heart failure. Neither intravenous nor oral itraconazole should
be used to treat infections in patients with evidence of ventricular
dysfunction unless the expected benefit clearly exceeds
the risk. Patients with risk factors for congestive heart failure
should be treated with caution and their condition monitored.
Veterinary Drugs and Treatments
Itraconazole may have use in veterinary medicine in the treatment
of systemic mycoses, including aspergillosis, cryptococcal meningitis,
blastomycosis, and histoplasmosis. Itraconazole
is probably
more effective than ketoconazole, but is significantly more expensive.
It may also be useful for superficial candidiasis or dermatophytosis,Itraconazole does not have appreciable effects (unlike ketoconazole)
on hormone synthesis and may have fewer side effects
than ketoconazole in small animals.
It is considered by many to be the drug of choice for treating
blastomycosis, unless moderate or severe
hypoxemia is present
(than amphotericin B).
In horses, itraconazole may be useful in the treatment of sporotrichosis
and Coccidioides immitis osteomyelitis.
in vitro
itraconazole was metabolized into hydroxy-itraconazole (oh-itz), a known in vivo metabolite of itz, and two new metabolites: keto-itraconazole (keto-itz) and n-desalkyl-itraconazole (nd-itz). itraconazole was a substrate for cyp3a and to characterize the metabolites generated. itraconazole exhibited an unbound km of 3.9 nm for cyp3a. itraconazole metabolites are as potent as or more potent cyp3a4 inhibitors than itz itself [1]. itraconazole was pharmacologically distinct from other azole antifungal agents. itraconazole has been shown to inhibit both the hedgehog signaling pathway and angiogenesis [2] itraconazole was active against 60 clinical isolates of aspergillus spp. with geometric mean (gm) mics of 0.25 mg/ml [3]. itraconazoleshowed an affinity for mammalian cytochrome p-450 enzymes as well as for fungal p-450-dependent enzyme, and thus has the potential for clinically important interactions [4].
in vivo
oral administration of itraconazole (200 mg) once daily for 4 days increased the area under the midazolam concentration-time curve from 10 to 15 times (p < 0.001) and mean peak concentrations three to four times (p < 0.001) compared with the placebo phase [5].
Usage
Itraconazole is available as oral capsules or oral solution. Please note that the capsules and solution are not interchangeable.
For effective treatment or prevention of fungal infections, please take the medication as instructed by your physician or pharmacist.
This drug does not work immediately. It may take weeks to months before you notice the benefits.
If you miss a dose, take the missed dose as soon as you remember. If it is almost time for your next dose, take only the usual dose. Do not double the dosage.
Metabolism
Itraconazole is extensively metabolized by the liver into a large number of metabolites, including hydroxyitraconazole, the major metabolite. The main metabolic pathways are oxidative scission of the dioxolane ring, aliphatic oxidation at the 1-methylpropyl substituent, N-dealkylation of this 1-methylpropyl substituent, oxidative degradation of the piperazine ring and triazolone scission.
Storage
Store at -20°C
References
1) Vanden Bossche?et al.?(1993),?Effects of itraconazole on cytochrome P-450-dependent sterol 14 alpha-demethylation and reduction of 3-ketosteroids in Cryptococcus neoformans; Antimicrob. Agents Chemother.,?37?2101 2) Liu?et al.?(2014),?Itraconazole suppresses the growth of glioblastoma through induction of autophagy: involvement of abnormal cholesterol trafficking; Autophagy,?10?1241 3) Kim?et al. (2010),?Itraconazole, a commonly used antifungal that inhibits Hedgehog pathway activity and cancer growth; Cancer Cell,?17?388 4) Nacev?et al. (2011),?The antifungal drug itraconazole inhibits vascular endothelial growth factor receptor 2 (VEGFR2) glycosylation, trafficking, and signaling in endothelial cells; J. Biol. Chem.,?286?44045
Properties of Itraconazole
| Melting point: | 166°C |
| Boiling point: | 850.0±75.0 °C(Predicted) |
| alpha | -0.1~+0.1°(D/20℃)(c=10,CH2Cl2) |
| Density | 1.27 g/cm3 |
| Flash point: | >110°(230°F) |
| storage temp. | 2-8°C |
| solubility | chloroform: 50 mg/mL, clear, colorless |
| form | White powder |
| pka | 3.7(at 25℃) |
| color | white |
| Water Solubility | Insoluble in water. Solube in chloroform at 50 mg/ml. Slightly soluble in ethanol or methanol
|
| Merck | 14,5245 |
| Stability: | Stable. Incompatible with strong oxidizing agents. |
| CAS DataBase Reference | 84625-61-6(CAS DataBase Reference) |
Safety information for Itraconazole
| Signal word | Warning |
| Pictogram(s) |
 Exclamation Mark Irritant GHS07 |
| GHS Hazard Statements |
H302:Acute toxicity,oral H315:Skin corrosion/irritation H319:Serious eye damage/eye irritation H335:Specific target organ toxicity, single exposure;Respiratory tract irritation |
| Precautionary Statement Codes |
P261:Avoid breathing dust/fume/gas/mist/vapours/spray. P264:Wash hands thoroughly after handling. P264:Wash skin thouroughly after handling. P270:Do not eat, drink or smoke when using this product. P301+P312:IF SWALLOWED: call a POISON CENTER or doctor/physician IF you feel unwell. P302+P352:IF ON SKIN: wash with plenty of soap and water. P305+P351+P338:IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. Continuerinsing. |
Computed Descriptors for Itraconazole
| InChIKey | VHVPQPYKVGDNFY-UHFFFAOYSA-N |
Itraconazole manufacturer
KP INNOVATIVE
New Products
4,4-Difluoropiperidine hydrochloride tert-butyl 9-methoxy-3-azaspiro[5.5]undecane-3-carboxylate Indole Methyl Resin N-Isopropylurea N,N-Dicyclohexylcarbodiimide(DCC) MELDRUMS ACID 5-METHYLISOXAZOLE-4-CARBOXYLIC ACID Magnessium Bis glycinate Zinc ascorbate 1-bromo-2-butyne 2-acetamidophenol 9(10H)-anthracenone Erythrosin B, 4-Piperidinopiperidine 2-((4-morpholinophenylamino) (methylthio) methylene) malononitrile 2,4-dihydroxybenzaldehyde 3-(4-morpholinophenylamino)-5-amino-1H-pyrazole-4-carbonitrile Methyl 2-methylquinoline-6-carboxylate 2,6-dichloro-4-nitropyridine 4-Bromo-2-chlorobenzonitrile 2-(benzylamino)acetic acid hydrochloride 4-(tert-Butoxycarbonylamino)but- 2-ynoic acid 3,4-dihydro-2H-benzo[b][1,4]dioxepine 1-Phenyl-1-cycloprppanecarboxylicacidRelated products of tetrahydrofuran
![8-[(E)-2-(3,4-dimethoxyphenyl)ethenyl]-1,3-diethyl-7-methyl-purine-2,6 -dione](https://img.chemicalbook.in/CAS/GIF/155270-99-8.gif)
![2,4-Dihydro-4-[4-[4-(4-hydroxyphenyl)-1-piperazinyl]phenyl]-](https://img.chemicalbook.in/CAS/GIF/79538-90-2.gif)
![1-[4-[[(2R,4S)-2-(2,4-Dichlorophenyl)-2-(1H-1,2,4-triazol-1-ylMethyl)-1,3-dioxolan-4-yl]Methoxy]phenyl]-4-(4-nitrophenyl)piperazine](https://img.chemicalbook.in/CAS/20150408/GIF/1437468-92-2.gif)
You may like
-
 Itraconazole 99%View Details
Itraconazole 99%View Details -
 Itraconazole 98%View Details
Itraconazole 98%View Details -
 Itraconazole 98%View Details
Itraconazole 98%View Details -
 Itraconazole 98%View Details
Itraconazole 98%View Details -
 Itraconazole 98%View Details
Itraconazole 98%View Details -
 Itraconazole 99%View Details
Itraconazole 99%View Details -
 Itraconazole BP/ EPView Details
Itraconazole BP/ EPView Details
84625-61-6 -
 Itraconazole Powder Api, Greater than 99%, IPView Details
Itraconazole Powder Api, Greater than 99%, IPView Details
84625-61-6
