Oxygen difluoride
- CAS NO.:7783-41-7
- Empirical Formula: F2O
- Molecular Weight: 54
- EINECS: 231-996-7
- SAFETY DATA SHEET (SDS)
- Update Date: 2024-12-18 14:15:30
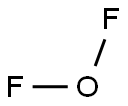
What is Oxygen difluoride?
Description
Oxygen difluoride is a colorless gas with afoul, peculiar odor. Shipped as a nonliquefied compressedgas. Molecular weight= 54.00; Boiling point=-145.6℃;Freezing/Melting point =-223.9℃; Vapor pressure =>1 atm at 20℃; Relative vapor density (air = 1) 5 1.88.Slightly soluble in water (slowly reactive); solubility =0.02%.
Chemical properties
Oxygen difluoride is a colorless gas. Foul, peculiar odor. Shipped as a nonliquefied compressed gas.
Physical properties
Colorless gas with a characteristic odor; unstable in the presence of moisture, otherwise stable up to 250°C; gas density 2.21g/L at 25°C; liquefies to a yellowish-brown liquid at -144.8°C; density of the liquid 1.90g/ml at -223.8°C; solidifies at -223.8°C; slightly soluble in water, decomposing very slowly; solubility 68ml gas per liter of water at 0°C; slightly soluble in acids and alkali.
The Uses of Oxygen difluoride
Commercial applications of oxygen difluoride are limited. It is used in organic synthesis to prepare fluoropropylenes and acylfluorides. It is used as an oxidizing and fluorinating agent in many preparative reactions and as a monomer in diolefin copolymerization.
The Uses of Oxygen difluoride
Oxidant in missile propellant systems
Preparation
Oxygen difluoride may be prepared by passing fluorine gas slowly through a dilute solution of caustic soda. Usually a 2% solution of NaOH is suitable for the preparation:
2F2 + 2 Na OH → 2NaF + OF2 + H2O
At a higher alkali concentration, oxygen is formed:
2F2 + 4NaOH → 4NaF + 2H2O + O2
Oxygen difluoride can be produced by electrolysis of an aqueous solution of HF or, alternatively, electroylzing molten potassium hydrogen difluoride, KHF2, in the presence of water.
Definition
ChEBI: Oxygen difluoride is an oxygen halide.
General Description
A colorless poisonous gas with a strong peculiar odor. Highly toxic by inhalation. Corrosive to skin and eyes. Can explode on contact with water. Decomposes to toxic gaseous fluorine if heated to high temperature. Prolonged exposure of the containers to high heat may result in their violent rupturing and rocketing. Used as an oxidizer for propellants.
Air & Water Reactions
Violent explosions resulted when a spark was discharged in a 25-70% mixture of Oxygen difluoride with oxygen over water [Mellor 2, Supp. 1:191. 1956].
Reactivity Profile
Oxygen difluoride is an oxidizing agent. Mixtures with carbon monoxide, with hydrogen, or with methane explode on sparking [Streng, A. G., Chem. Rev., 1963, 63, p. 610]. May react explosively with adsorbents (silica, alumina, molecular sieves, silica gel) [Streng A. G., Chem. Eng. News, 1965, 43(12), p. 5]; the presence of moisture may render such mixtures shock-sensitive [Metz, F. I., Chem. Eng. News, 1965, 43(7), p. 41]. Gives explosive reactions with diborane, hydrogen sulfide, nitrogen oxide, nitrosyl fluoride, charcoal, sulfur tetrafluoride. Warming of mixtures with halogens, with metal halides, with aluminum chloride, with antimony pentachloride, and with tungsten gives explosions. Ignites on contact with diborane tetrafluoride, phosphorus pentaoxide, red phosphorus, boron, silicon [Bretherick, 5th ed., 1995, p. 1419]. Incompatible with ammonia, arsenic trioxide, chromium trioxide, chlorine in the presence of copper, ozone [Lewis, 3rd ed., 1993, p. 978]. Reacts to incandescence with aluminum, barium, cadmium, magnesium, strontium, zinc, zirconium. Reacts with the alkali metals (lithium, sodium, potassium) [Streng, A. G., Chem. Rev., 1963, 63, p. 611].
Hazard
Oxygen difluoride is a highly toxic gas that attacks lungs, manifesting delayed symptoms. It causes irritation of eyes, lungs, and skin. Chronic exposure can lead to pulminary edema and congestion in lungs. Inhalation also can cause systemic toxic effects in humans. LC50 inhalation (rat): 136ppm/1 hr The compound is a very powerful oxidizing agent. Contact with reducing agents can cause explosion.
Health Hazard
TOXIC; may be fatal if inhaled or absorbed through skin. Fire will produce irritating, corrosive and/or toxic gases. Contact with gas or liquefied gas may cause burns, severe injury and/or frostbite. Runoff from fire control may cause pollution.
Fire Hazard
Substance does not burn but will support combustion. Vapors from liquefied gas are initially heavier than air and spread along ground. These are strong oxidizers and will react vigorously or explosively with many materials including fuels. May ignite combustibles (wood, paper, oil, clothing, etc.). Some will react violently with air, moist air and/or water. Cylinders exposed to fire may vent and release toxic and/or corrosive gas through pressure relief devices. Containers may explode when heated. Ruptured cylinders may rocket.
Safety Profile
Poison by inhalation. Human systemic effects by inhalation: chronic pulmonary edema or congestion. A corrosive skin, eye, and mucous membrane irritant. Attacks lungs with delayed appearance of symptoms. A very powerful oxidizer. Must be kept away from contact with reducing agents. Explosive reaction with adsorbents (e.g., sllica gel, alumina, molecular sieve), diborane, halogens + heat, metal halides, aluminum chloride, antimony pentachloride (at 1 50℃), tungsten + heat, hydrogen sulfide, liquid nitrogen oxide, nitrosyl fluoride, charcoal, sulfur tetrafluoride. Forms spark-sensitive explosive mixtures with water or combustible gases (e.g., carbon monoxide, hydrogen, methane). Ignites on contact with diborane tetrafluoride, nonmetals (e.g., red phosphorus, boron powder, silicon), phosphorus(V) oxide, nitrogen oxide gas. Incandescent reaction with metals (e.g., aluminum, barium, cadmium, magnesium, strontium, zinc, zirconium, lithium (above 4OO0C)), potassium (above 4OO0C), sodium. Incompatible with NH3, As203, Cl2 + Cu, CrO3, Ir, 03, O2 + H20, Pd, Pt, Rh, Ru, Si02. When heated to decomposition it emits highly toxic fumes of F-. See also FLUORIDES
Potential Exposure
Oxygen difluoride is used as an oxidizer in missile propellant systems.
First aid
If this chemical gets into the eyes, remove anycontact lenses at once and irrigate immediately for at least15 min, occasionally lifting upper and lower lids. Seek medical attention immediately. If this chemical contacts theskin, remove contaminated clothing and wash immediatelywith soap and water. Seek medical attention immediately. Ifthis chemical has been inhaled, remove from exposure,begin rescue breathing (using universal precautions, including resuscitation mask) if breathing has stopped and CPR ifheart action has stopped. Transfer promptly to a medicalfacility. When this chemical has been swallowed, get medical attention. Give large quantities of water and inducevomiting. Do not make an unconscious person vomit.Medical observation is recommended for 24-48 h afterbreathing overexposure, as pulmonary edema may bedelayed. As first aid for pulmonary edema, a doctor orauthorized paramedic may consider administering a corticosteroid spray. If frostbite has occurred, seek medical attention immediately; do NOT rub the affected areas or flushthem with water. In order to prevent further tissue damage,do NOT attempt to remove frozen clothing from frostbittenareas. If frostbite has NOT occurred, immediately and thoroughly wash contaminated skin with soap and water.
storage
Color Code—Yellow Stripe: Reactivity Hazard;Store separately in an area isolated from flammables, combustibles, or other yellow-coded materials. Prior to workingwith this chemical you should be trained on its proper handling and storage. Protect cylinder containers against physical damage. Do not use wood pallets. Preferably handlebehind body shield, in outdoor, or open protective fences.Wear long rubber gloves, goggles, protective clothing, andself-contained breathing apparatus. See 29 CFR 1910.101for specific regulations on storage of compressed gas. SeeOSHA Standard 1910.104 and NFPA 43A Code for theStorage of Liquid and Solid Oxidizers for detailed handlingand storage regulations
Shipping
Oxygen difluoride, compressed Hazard Class: 2.3; Labels: 2.3-Poisonous gas, 5.1-Oxidizer, 8-Corrosive material, Inhalation Hazard Zone A. Cylinders must be transported in a secure upright position, in a well-ventilated truck. Protect cylinder and labels from physical damage. The owner of the compressed gas cylinder is the only entity allowed by federal law (49CFR) to transport and refill them. It is a violation of transportation regulations to refill compressed gas cylinders without the express written permission of the owner.
Incompatibilities
A strong oxidizer. Explodes on contact with steam. Violent reaction with reducing agents; combustible materials; chlorine, bromine, iodine, platinum, metal oxides; moist air; hydrogen sulfide (explosive in ambient air); hydrocarbons, water. Attacks mercury. Reacts, possibly violently, with many materials including porous materials (i.e., alumina, charcoal, and silica), mercury, and phosphorus.
Waste Disposal
Spray or sift on a thick layer of a (1:1) mixture of dry soda ash and slaked lime behind ashield. After mixing, spray water from an atomizer with great precaution. Transfer slowly into a large amount of water. Neutralize and drain into the sewer with sufficient water.
Properties of Oxygen difluoride
| Melting point: | -223.8° |
| Boiling point: | bp -145.3° |
| Density | (liq; -224°) 1.90 |
| solubility | slightly soluble in H2O |
| form | colorless gas |
| color | Colorless gas or yellowish-brown liquid |
| Water Solubility | 6.8mL gas/100mL H2O (0°C) [MER06] |
| EPA Substance Registry System | Oxygen difluoride (7783-41-7) |
Safety information for Oxygen difluoride
Computed Descriptors for Oxygen difluoride
New Products
Tert-butyl bis(2-chloroethyl)carbamate (S)-3-Aminobutanenitrile hydrochloride N-Boc-D-alaninol N-BOC-D/L-ALANINOL N-octanoyl benzotriazole 4-Hydrazinobenzoic acid 3,4-Dibenzyloxybenzaldehyde Electrolytic Iron Powder 1,1’-CARBONYLDIIMIDAZOLE R-2-BENZYLOXY PROPIONIC ACID 4-HYDROXY BENZYL ALCOHOL 1,1’-CARBONYLDI (1,2-4 TRIAZOLE) S-2-CHLORO PROPIONIC ACID (2-Hydroxyphenyl)acetonitrile 4-Bromopyrazole 5-BROMO-2CYANO PYRIDINE 5,6-Dimethoxyindanone 5-broMo-2-chloro-N-cyclopentylpyriMidin-4-aMine 3-(Hydroxymethyl)benzoate N-Boc-2-chloroethylamine 1-Bromo-2-methoxy-3-nitrobenzene N-Methyl-3-cyclopenten-1-amine 2-Bromo-3-hydroxybenzaldehyde 1H-indazole-5-carboxamideRelated products of tetrahydrofuran
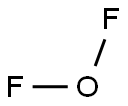
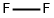
You may like
-
 7441-43-2 98%View Details
7441-43-2 98%View Details
7441-43-2 -
 1260741-78-3 6-Bromo-3-iodo-1-methyl-1H-indazole 98%View Details
1260741-78-3 6-Bromo-3-iodo-1-methyl-1H-indazole 98%View Details
1260741-78-3 -
 (3-Benzyloxypropyl)triphenyl phosphonium 98%View Details
(3-Benzyloxypropyl)triphenyl phosphonium 98%View Details
54314-85-1 -
 4-bromo-3,5-dimethylbenzenesulfonyl chloride 1581266-79-6 98%View Details
4-bromo-3,5-dimethylbenzenesulfonyl chloride 1581266-79-6 98%View Details
1581266-79-6 -
 2490430-37-8 98%View Details
2490430-37-8 98%View Details
2490430-37-8 -
 N-(5-Amino-2-methylphenyl)acetamide 5434-30-0 98%View Details
N-(5-Amino-2-methylphenyl)acetamide 5434-30-0 98%View Details
5434-30-0 -
 124371-59-1 98%View Details
124371-59-1 98%View Details
124371-59-1 -
 53857-52-2 98%View Details
53857-52-2 98%View Details
53857-52-2