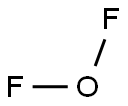CHEMICAL AND PHYSICAL PROPERTIES
| Physical Description | Oxygen difluoride appears as a colorless poisonous gas with a strong peculiar odor. Highly toxic by inhalation. Corrosive to skin and eyes. Can explode on contact with water. Decomposes to toxic gaseous fluorine if heated to high temperature. Prolonged exposure of the containers to high heat may result in their violent rupturing and rocketing. Used as an oxidizer for propellants. |
|---|---|
| Color/Form | COLORLESS GAS; YELLOWISH-BROWN WHEN LIQUID |
| Odor | PECULIAR SMELL |
| Boiling Point | -230 °F at 760 mmHg (NIOSH, 2023) |
| Melting Point | -371 °F (NIOSH, 2023) |
| Solubility | 0.02 % (NIOSH, 2023) |
| Density | 1.90 at -224 °C (LIQ) |
| Vapor Density | 1.88 (NIOSH, 2023) - Heavier than air; will sink (Relative to Air) |
| Vapor Pressure | greater than 1 atm (NIOSH, 2023) |
| Stability/Shelf Life | GAS MAY BE KEPT OVER WATER UNCHANGED FOR A MONTH |
| Decomposition | WHEN HEATED TO DECOMPOSITION, IT EMITS HIGHLY TOXIC FUMES OF ... /HYDROGEN FLUORIDE/. |
| Corrosivity | DOES NOT ATTACK GLASS IN COLD; CORRODES MERCURY |
| Ionization Potential | 13.11 eV |
| Odor Threshold | Odor Threshold Low: 0.09 [ppm] Odor threshold from AIHA |
| Other Experimental Properties | TROUTON CONSTANT: 20.65; REACTS VERY SLOWLY WITH WATER; GAS MAY BE KEPT OVER WATER UNCHANGED FOR A MONTH |
| Chemical Classes | Toxic Gases & Vapors -> Oxidizers |
COMPUTED DESCRIPTORS
| Molecular Weight | 53.996 g/mol |
|---|---|
| XLogP3 | 1.2 |
| Hydrogen Bond Donor Count | 0 |
| Hydrogen Bond Acceptor Count | 3 |
| Rotatable Bond Count | 0 |
| Exact Mass | 53.99172094 g/mol |
| Monoisotopic Mass | 53.99172094 g/mol |
| Topological Polar Surface Area | 9.2 Ų |
| Heavy Atom Count | 3 |
| Formal Charge | 0 |
| Complexity | 2.8 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 0 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently-Bonded Unit Count | 1 |
| Compound Is Canonicalized | Yes |
PRODUCT INTRODUCTION
description
Oxygen difluoride appears as a colorless poisonous gas with a strong peculiar odor. Highly toxic by inhalation. Corrosive to skin and eyes. Can explode on contact with water. Decomposes to toxic gaseous fluorine if heated to high temperature. Prolonged exposure of the containers to high heat may result in their violent rupturing and rocketing. Used as an oxidizer for propellants.