Phosphorus trioxide
- CAS NO.:1314-24-5
- Empirical Formula: H4O5P2
- Molecular Weight: 145.976282
- MDL number: MFCD09970509
- SAFETY DATA SHEET (SDS)
- Update Date: 2024-12-18 14:07:02
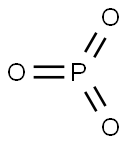
What is Phosphorus trioxide?
Chemical properties
transparent crystal(s); monoclinic or colorless liquid; disproportionates to red P and P2O4if heated above 210°C [MER06]
General Description
White crystalline solid or a liquid (melting point 24°C). Density 2.14 g / cm3. Toxic and corrosive. May severely irritate skin and eyes. Used to make other chemicals.
Air & Water Reactions
Fumes in air. Reacts slowly with cold water to give phosphorous acid. Reacts vigorously with hot water to generate red phosphorus, phosphine (highly toxic and flammable) and phosphoric acid [Merck 11th ed. 1989].
Reactivity Profile
Phosphorus trioxide reacts exothermically with bases. Melted Phosphorus trioxide readily ignites in air. Instantly ignites with a flame of almost blinding brilliance when thrown into oxygen at 50-60°C [J. Am. Chem. Soc. 125:347. 1924]. Incompatible with ammonia, disulfur dichloride, halogens, oxygen, phosphorus pentachloride, sulfur, sulfuric acid, water [Bretherick, 5th ed., 1995, p. 1778].
Health Hazard
TOXIC; inhalation, ingestion or contact (skin, eyes) with vapors, dusts or substance may cause severe injury, burns or death. Reaction with water or moist air will release toxic, corrosive or flammable gases. Reaction with water may generate much heat that will increase the concentration of fumes in the air. Fire will produce irritating, corrosive and/or toxic gases. Runoff from fire control or dilution water may be corrosive and/or toxic and cause pollution.
Fire Hazard
Non-combustible, substance itself does not burn but may decompose upon heating to produce corrosive and/or toxic fumes. Vapors may accumulate in confined areas (basement, tanks, hopper/tank cars etc.). Substance will react with water (some violently), releasing corrosive and/or toxic gases and runoff. Contact with metals may evolve flammable hydrogen gas. Containers may explode when heated or if contaminated with water.
Properties of Phosphorus trioxide
| Melting point: | 23.8° |
| Boiling point: | bp 173.1° in nitrogen atm |
| Density | d421 2.135 |
| solubility | reacts with H2O |
| form | colorless monoclinic crystals or liquid |
| color | colorless monoclinic crystals, crystalline or
liquid |
| Water Solubility | slowly forms H3PO3 in cold H2O; reacts violently in hot H2O forming red P, PH3, and H3PO4 [MER06] |
| EPA Substance Registry System | Phosphorus trioxide (1314-24-5) |
Safety information for Phosphorus trioxide
Computed Descriptors for Phosphorus trioxide
New Products
(S)-3-Aminobutanenitrile hydrochloride 4-Methylphenylacetic acid N-Boc-D-alaninol N-BOC-D/L-ALANINOL Tert-butyl bis(2-chloroethyl)carbamate 3-Morpholino-1-(4-nitrophenyl)-5,6-dihydropyridin- 2(1H)-one Furan-2,5-Dicarboxylic Acid Tropic acid 1-Bromo-3,5-Di-Tert-Butylbenzene S-2-CHLORO PROPIONIC ACID ETHYL ISOCYANOACETATE 2-Bromo-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate 4-IODO BENZOIC ACID 3-NITRO-2-METHYL ANILINE 1-(2,4-DICHLOROPHENYL) ETHANAMINE (2-Hydroxyphenyl)acetonitrile 4-Bromopyrazole 2-(Cyanocyclohexyl)acetic acid 4-methoxy-3,5-dinitropyridine 1-(4-(aminomethyl)benzyl)urea hydrochloride 2-aminopropyl benzoate hydrochloride diethyl 2-(2-((tertbutoxycarbonyl)amino) ethyl)malonate tert-butyl 4- (ureidomethyl)benzylcarbamate Ethyl-2-chloro((4-methoxyphenyl)hydrazono)acetateRelated products of tetrahydrofuran
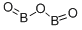
You may like
-
 2033-24-1 98%View Details
2033-24-1 98%View Details
2033-24-1 -
 42831-50-5 5-METHYLISOXAZOLE-4-CARBOXYLIC ACID 98%View Details
42831-50-5 5-METHYLISOXAZOLE-4-CARBOXYLIC ACID 98%View Details
42831-50-5 -
 1975-50-4 98%View Details
1975-50-4 98%View Details
1975-50-4 -
 2-HYDROXY BENZYL ALCOHOL 98%View Details
2-HYDROXY BENZYL ALCOHOL 98%View Details
90-01-7 -
 2-Chloro-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate 221615-75-4 98%View Details
2-Chloro-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate 221615-75-4 98%View Details
221615-75-4 -
 61397-56-6 CIS BROMO BENZOATE 98%View Details
61397-56-6 CIS BROMO BENZOATE 98%View Details
61397-56-6 -
 14714-50-2 (2-Hydroxyphenyl)acetonitrile 98+View Details
14714-50-2 (2-Hydroxyphenyl)acetonitrile 98+View Details
14714-50-2 -
 118753-70-1 98+View Details
118753-70-1 98+View Details
118753-70-1