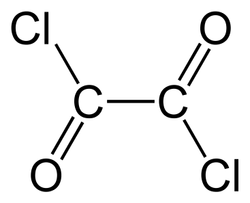
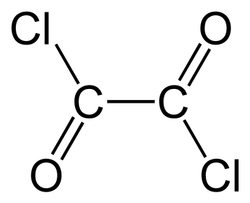
Oxalyl chloride
Synonym(s):Ethanedioyl dichloride;Oxalic acid dichloride;Oxalyl chloride
- CAS NO.:79-37-8
- Empirical Formula: C2Cl2O2
- Molecular Weight: 126.93
- MDL number: MFCD00000704
- EINECS: 201-200-2
- SAFETY DATA SHEET (SDS)
- Update Date: 2025-09-25 17:15:13
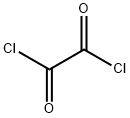
What is Oxalyl chloride?
Chemical properties
colourless liquid with a pungent odour
The Uses of Oxalyl chloride
Oxalyl Chloride is used in the preparation of cardiolipin analogs which induce peroxidase activity by Cytochrome C. It is also used in the preparation of fluorescent indicators for cytosolic calcium.
The Uses of Oxalyl chloride

To a suspension of the SM (0.3 g, 2.38 mmol) in DCM (10 mL) was added (COCl)2 (0.3 mL, 3.6 mmol) dropwise at 0 C. The resulting mixture was stirred at RT for 1 h then heated to 70 C for 2 h. After cooling to RT the excess (COCl)2 and solvent were removed in vacuo (at <40 C) to provide the product as a gum which was taken to the next step without further purification.
Production Methods
Oxalyl Chloride is produced by the reaction of anhydrous oxalic acid and phosphorus pentachloride.
Preparation
Oxalyl chloride was first prepared in 1892 by the French chemist Adrien Fauconnier, who reacted diethyl oxalate with phosphorus pentachloride. Oxalyl chloride is also produced commercially from ethylene carbonate. Photochlorination gives the tetrachloride, which is subsequently degraded:
C2H4O2CO + 4 Cl2 → C2Cl4O2CO + 4 HCl
C2Cl4O2CO → C2O2Cl2 + COCl2
What are the applications of Application
Oxalyl chloride is used in organic synthesis. It may be used in the following processes:
Unncatalyzed reaction of silyl ketene acetals with oxalyl chloride yeilds symmetrical pulvinic acids.
Catalytic syntheses of N-heterocyclic ynones and ynediones by in situ activation of carboxylic acids with oxalyl chloride.
Oxalyl chloride was reportedly used in the first synthesis of dioxane tetraketone.
Preparation of Mosher′s acid chloride by reacting with Mosher′s acid in the presence of DMF.
Activation of dimethyl sulfoxide for use in the oxidation of long-chain alcohols to carbonyls.
Activation of α-keto carboxylic acids and N-heterocyclic carboxylic acids for alkynylation to form ynediones and N-heterocyclic ynones, respectively.
Reactions
Oxalyl Chloride vigorously reacts with water, alcohols, and amines, and is employed for the synthesis of agrochemicals, pharmaceuticals, and fine chemicals.
General Description
Oxalyl chloride is a commonly used chlorinating reagent that can be prepared by the reaction of oxalic acid and phosphorus pentachloride.
Health Hazard
Oxalyl chloride is a corrosive respiratory irritant and lachrymator. The vapors will attack the skin, eyes and especially the mucous membranes of the nose and throat and respiratory system. This material should be used only in a well ventilated area.
Fire Hazard
Extinguish with dry powder or carbon dioxide. Do not use water. Oxalyl chloride decomposes upon contact with water to produce toxic and corrosive fumes. When heated to decomposition, product emits toxic fumes.
Chemical Reactivity
Product is sensitive to temperatures below -10° and above 40°C. It reacts vigorously with water and hydroxyl compounds.
Safety Profile
Poison. Violently decomposed by water and alcohol. Severe irritant to skin, eyes, respiratory tract. Explodeson contact with dimethyl sulfoxide. Forms shock-sensitive explosive mixtures with potassium or with K-Na alloy. Will react with water or steam to
Waste Disposal
Carefully mix acidic compound with dry sodium bicarbonate. Dilute slowly with water and wash down the drain with copious amounts of water.
Properties of Oxalyl chloride
| Melting point: | -10--8 °C (lit.) |
| Boiling point: | 62-65 °C (lit.) |
| Density | 1.5 g/mL at 20 °C (lit.) |
| vapor density | 4.4 (vs air) |
| vapor pressure | 150 mm Hg ( 20 °C) |
| refractive index | n |
| Flash point: | 176-178°C |
| storage temp. | Store Cold |
| solubility | Chloroform (Soluble), Ethyl Acetate |
| form | Liquid |
| appearance | Colorless liquid |
| color | APHA: 0-150 |
| Odor | penetrating odor |
| Water Solubility | reacts |
| Sensitive | Moisture Sensitive |
| Decomposition | 176-178 ºC |
| Merck | 14,6914 |
| BRN | 1361988 |
| Exposure limits | ACGIH: TWA 50 ppm OSHA: TWA 25 ppm; STEL 125 ppm NIOSH: IDLH 2300 ppm |
| Dielectric constant | 3.5(21℃) |
| Stability: | Stable. Incompatible with bases, alcohols, steel, oxidizing agents, alkali metals. Moisture sensitive. Reacts violently with water, liberating toxic gas. |
| CAS DataBase Reference | 79-37-8(CAS DataBase Reference) |
| NIST Chemistry Reference | Oxalyl chloride(79-37-8) |
| EPA Substance Registry System | Ethanedioyl dichloride (79-37-8) |
Safety information for Oxalyl chloride
| Signal word | Danger |
| Pictogram(s) |
 Flame Flammables GHS02  Corrosion Corrosives GHS05  Skull and Crossbones Acute Toxicity GHS06 |
| GHS Hazard Statements |
H260:Substances And Mixtures Which, In Contact With Water,Emit Flammable Gases H314:Skin corrosion/irritation |
| Precautionary Statement Codes |
P223:Keep away from any possible contact with water, because of violent reaction and possible flash fire. P280:Wear protective gloves/protective clothing/eye protection/face protection. P231+P232:Handle under inert gas. Protect from moisture. P303+P361+P353:IF ON SKIN (or hair): Remove/Take off Immediately all contaminated clothing. Rinse SKIN with water/shower. P305+P351+P338:IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. Continuerinsing. |
Computed Descriptors for Oxalyl chloride
| InChIKey | CTSLXHKWHWQRSH-UHFFFAOYSA-N |
Oxalyl chloride manufacturer
JSK Chemicals
VJ CHEMTRADE PVT LTD
New Products
4,4-Difluoropiperidine hydrochloride tert-butyl 9-methoxy-3-azaspiro[5.5]undecane-3-carboxylate Indole Methyl Resin N-Isopropylurea N,N-Dicyclohexylcarbodiimide(DCC) MELDRUMS ACID 5-METHYLISOXAZOLE-4-CARBOXYLIC ACID Magnessium Bis glycinate Zinc ascorbate 1-bromo-2-butyne 2-acetamidophenol 9(10H)-anthracenone Erythrosin B, 4-Piperidinopiperidine 2-((4-morpholinophenylamino) (methylthio) methylene) malononitrile 2,4-dihydroxybenzaldehyde 3-(4-morpholinophenylamino)-5-amino-1H-pyrazole-4-carbonitrile Methyl 2-methylquinoline-6-carboxylate 2,6-dichloro-4-nitropyridine 4-Bromo-2-chlorobenzonitrile 2-(benzylamino)acetic acid hydrochloride 4-(tert-Butoxycarbonylamino)but- 2-ynoic acid 3,4-dihydro-2H-benzo[b][1,4]dioxepine 1-Phenyl-1-cycloprppanecarboxylicacidRelated products of tetrahydrofuran

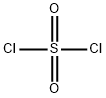
You may like
-
 Oxalyl chloride 98% supplierView Details
Oxalyl chloride 98% supplierView Details
79-37-8 -
 Oxalyl Chloride 98%View Details
Oxalyl Chloride 98%View Details -
 Oxalyl Chloride CASView Details
Oxalyl Chloride CASView Details -
 Oxalyl chloride CAS 79-37-8View Details
Oxalyl chloride CAS 79-37-8View Details
79-37-8 -
 Oxalyl chloride 98% CAS 79-37-8View Details
Oxalyl chloride 98% CAS 79-37-8View Details
79-37-8 -
 Oxalyl Chloride CAS No 79-37-8, For Industrial, 50 KgView Details
Oxalyl Chloride CAS No 79-37-8, For Industrial, 50 KgView Details
79-37-8 -
 99% Oxaly Chloride, 250kg drumView Details
99% Oxaly Chloride, 250kg drumView Details
79-37-8 -
 OXALYL CHLORIDE INTERMEDIATEView Details
OXALYL CHLORIDE INTERMEDIATEView Details
79-37-8
