Terephthaloyl chloride
- CAS NO.:100-20-9
- Empirical Formula: C8H4Cl2O2
- Molecular Weight: 203.02
- MDL number: MFCD00000693
- EINECS: 202-829-5
- SAFETY DATA SHEET (SDS)
- Update Date: 2024-12-18 14:15:30
What is Terephthaloyl chloride?
Description
Terephthaloyl chloride (1,4-benzenedicarbonyl chloride), the acid chloride of terephthalic acid, is used primarily to make specialty polymers, primarily aromatic polyamides, or aramids. In 1965, inventors at DuPont developed the aramid Kevlar, a copolymer of terephthaloyl chloride and p-phenylenediamine. Its first use was as a replacement for steel in racing tires.
Chemical properties
monoclinic crystals or white crystalline flakes. Soluble in ethanol and organic solvents.
The Uses of Terephthaloyl chloride
Terephthaloyl chloride and Isophthaloyl dichloride have many similar uses as monomers for the synthesis of specialty fibers and as raw materials for polymers such as polyamides and polyesters. Dyemanufacture; synthetic fibers, resins, films; UV absorption; pharmaceuticals; rubber chemicals; cross-linking agent for polyurethanes and polysulfides.
The Uses of Terephthaloyl chloride
Terephthaloyl chloride acts as a monomer with p-phenylenediamine used in the preparation of polymer viz. poly paraphenylene terephthalamide. It is an active component in performance polymers and aramid fibers, which gives flame resistance, chemical resistance, temperature stability, light weight, and very high strength. It is also used as an effective water scavenger, utilized to stabilize isocyanates and urethane prepolymers. Further, it is used in the preparation of liquid crystalline thermosets by thermal cyclotrimerization of dicyanate compounds of ring substituted bis(4-hydroxyphenyl) terepthalates. In addition to this, it is involved in the condensation reaction with difunctional alfa, μ-diaminopolystyrene to get chain-extended polystyrene containing amide bonds along the polymer backbone.
Preparation
Synthesis of terephthaloyl chloride by thionyl chloride method: terephthalic acid is mixed with thionyl chloride, refluxed at 80℃ for 10-12h, then the thionyl chloride is evaporated, and the resulting crude product is distilled under reduced pressure to obtain the finished product.
General Description
Terephthaloyl chloride undergoes condensation reaction with difunctional α,ω-diaminopolystyrene to yield chain-extended polystyrene containing amide bonds along the polymer backbone. It undergoes interfacial reaction with bovine serum albumin to form thin cross-linked films.
Hazard
Skin irritant.
Flammability and Explosibility
Non flammable
Safety Profile
Moderately toxic by ingestion. Corrosive. Combustible when exposed to heat or flame. When heated to decomposition it emits toxic fumes of Cl-. See also CHLORIDES.
Purification Methods
Crystallise the acid chloride from dry hexane. [Beilstein 9 IV 3318.]
Properties of Terephthaloyl chloride
| Melting point: | 79-81 °C(lit.) |
| Boiling point: | 266 °C(lit.) |
| Density | 1,34 g/cm3 |
| vapor density | 7 (vs air) |
| vapor pressure | 0.02 mm Hg ( 25 °C) |
| refractive index | 1.5684 (estimate) |
| Flash point: | 356 °F |
| storage temp. | 2-8°C |
| solubility | ethanol: 5%, clear |
| form | flakes |
| color | White to Almost white |
| PH | <0.5 (H2O) |
| explosive limit | 1.5-8.9%(V) |
| Water Solubility | REACTS |
| Sensitive | Moisture Sensitive |
| BRN | 607796 |
| CAS DataBase Reference | 100-20-9(CAS DataBase Reference) |
| NIST Chemistry Reference | 1,4-Benzenedicarbonyl dichloride(100-20-9) |
| EPA Substance Registry System | 1,4-Benzenedicarbonyl dichloride (100-20-9) |
Safety information for Terephthaloyl chloride
| Signal word | Danger |
| Pictogram(s) |
 Corrosion Corrosives GHS05  Skull and Crossbones Acute Toxicity GHS06 |
| GHS Hazard Statements |
H314:Skin corrosion/irritation H331:Acute toxicity,inhalation H335:Specific target organ toxicity, single exposure;Respiratory tract irritation |
| Precautionary Statement Codes |
P260:Do not breathe dust/fume/gas/mist/vapours/spray. P271:Use only outdoors or in a well-ventilated area. P280:Wear protective gloves/protective clothing/eye protection/face protection. P303+P361+P353:IF ON SKIN (or hair): Remove/Take off Immediately all contaminated clothing. Rinse SKIN with water/shower. P305+P351+P338:IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. Continuerinsing. |
Computed Descriptors for Terephthaloyl chloride
| InChIKey | LXEJRKJRKIFVNY-UHFFFAOYSA-N |
Terephthaloyl chloride manufacturer
JSK Chemicals
Panoli Intermediates (India) Pvt., Limited. (Kutch Chemical Industries Ltd.)
Shree Sulphurics Pvt Ltd (SSPL)
Anand Agencies
New Products
(S)-3-Aminobutanenitrile hydrochloride 4-Methylphenylacetic acid N-Boc-D-alaninol N-BOC-D/L-ALANINOL Tert-butyl bis(2-chloroethyl)carbamate N-octanoyl benzotriazole 3-Morpholino-1-(4-nitrophenyl)-5,6-dihydropyridin- 2(1H)-one Furan-2,5-Dicarboxylic Acid S-2-CHLORO PROPIONIC ACID ETHYL ISOCYANOACETATE 2-Bromo-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate 4-IODO BENZOIC ACID 3-NITRO-2-METHYL ANILINE 1-(2,4-DICHLOROPHENYL) ETHANAMINE (2-Hydroxyphenyl)acetonitrile 4-Bromopyrazole 5,6-Dimethoxyindanone 2-(Cyanocyclohexyl)acetic acid 4-methoxy-3,5-dinitropyridine 1-(4-(aminomethyl)benzyl)urea hydrochloride 2-aminopropyl benzoate hydrochloride diethyl 2-(2-((tertbutoxycarbonyl)amino) ethyl)malonate tert-butyl 4- (ureidomethyl)benzylcarbamate Ethyl-2-chloro((4-methoxyphenyl)hydrazono)acetateRelated products of tetrahydrofuran

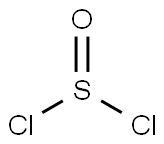
You may like
-
 100-20-9 Terephthaloyl chloride, 98% 99%View Details
100-20-9 Terephthaloyl chloride, 98% 99%View Details
100-20-9 -
 100-20-9 99%View Details
100-20-9 99%View Details
100-20-9 -
 Terephthaloyl Chloride pure CAS 100-20-9View Details
Terephthaloyl Chloride pure CAS 100-20-9View Details
100-20-9 -
 Terephthaloyl chloride CAS 100-20-9View Details
Terephthaloyl chloride CAS 100-20-9View Details
100-20-9 -
 Terephthaloyl Chloride CAS 100-20-9View Details
Terephthaloyl Chloride CAS 100-20-9View Details
100-20-9 -
 Terephthaloyl chloride 100-20-9 99%View Details
Terephthaloyl chloride 100-20-9 99%View Details
100-20-9 -
 Terphthaloyl chloride CAS 100-20-9View Details
Terphthaloyl chloride CAS 100-20-9View Details
100-20-9 -
 Terephthaloyl chloride CAS 100-20-9View Details
Terephthaloyl chloride CAS 100-20-9View Details
100-20-9