OLSALAZINE
- CAS NO.:15722-48-2
- Empirical Formula: C14H10N2O6
- Molecular Weight: 302.24
- MDL number: MFCD00602469
- EINECS: 605-089-5
- SAFETY DATA SHEET (SDS)
- Update Date: 2024-07-02 08:55:03
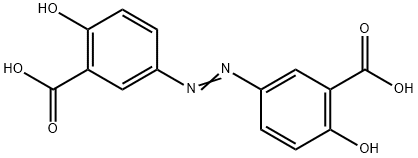
What is OLSALAZINE?
Absorption
After oral administration, olsalazine has limited systemic bioavailability, with less than 5% of the oral dose being absorbed. Based on oral and intravenous dosing studies, approximately 2.4% of a single 1 g oral dose is absorbed. Maximum serum concentrations of olsalazine appear after approximately one hour and, even after a 1 g single dose, are low (e.g., 1.6 to 6.2 μmol/L).
Patients on daily doses of 1 g olsalazine for two to four years show a stable plasma concentration of olsalazine-S (3.3 to 12.4 μmol/L). Olsalazine-S accumulates to a steady state within two to three weeks. Serum concentrations of mesalamine are detected after four to eight hours. The peak levels of mesalamine after an oral dose of 1 g olsalazine are low (i.e., 0 to 4.3 μmol/L).
Toxicity
There is no information available regarding acute toxicity (LD50) of olsalazine. Symptoms of salicylate toxicity include nausea, vomiting and abdominal pain, tachypnea, hyperpnea, tinnitus, and neurologic symptoms (headache, dizziness, confusion, seizures). Severe intoxication may lead to electrolyte and blood pH imbalance and potentially to organ damage. There is no antidote for olsalazine overdose; however, conventional therapy for salicylate toxicity, such as gastrointestinal tract decontamination, may be beneficial in acute overdosage. Overdose should be managed with proper medical care and appropriate supportive care, including the possible use of emesis, cathartics, and activated charcoal to prevent further absorption. Fluid and electrolyte imbalance may be managed with appropriate intravenous therapy and maintenance of adequate renal function.
The Uses of OLSALAZINE
Anti-inflammatory (gastrointestinal).
Indications
In the US, olsalazine is indicated for the maintenance of remission of ulcerative colitis in adult patients who are intolerant to sulfasalazine.
In Canada, it is used in the treatment of acute ulcerative colitis of mild to moderate severity, with or without the concomitant use of steroids. It is also indicated for the long-term maintenance of patients with ulcerative colitis in remission.
Background
Olsalazine is an aminosalicylate and a prodrug of mesalamine (5-aminosalicylic acid, 5-ASA). It was first developed for delivering mesalamine to the colon without the use of sulfapyridine. Olsalazine comprises two mesalamine molecules joined by an azo bridge, which is cleaved in the colon. Olsalazine is an anti-inflammatory agent that works by inhibiting cyclooxygenase and lipoxygenase, subsequently reducing the production of pro-inflammatory factors like prostaglandin and leukotriene. Olsalazine is used in the treatment of ulcerative colitis.
Definition
ChEBI: An azobenzene that consists of two molecules of 4-aminosalicylic acid joined by an azo linkage. A prodrug for mesalazine, an anti-inflammatory drug, it is used (as the disodium salt) in the treatment of inflammatory bowel disease.
Indications
Olsalazine is approved for maintenance of remission of ulcerative colitis, but a commonly reported side effect is a paradoxical increase in diarrhea. The U. S. Food and Drug Administration (FDA) has approved balsalazide disodium (Colazal) as a treatment of mild to moderately active ulcerative colitis.
brand name
Dipentum (UCB).
Mechanism of action
Olsalazine is approved for maintenance of remission of ulcerative colitis, but a commonly reported side effect is a paradoxical increase in diarrhea. The U. S. Food and Drug Administration (FDA) has approved balsalazide disodium (Colazal) as a treatment of mild to moderately active ulcerative colitis.
Pharmacokinetics
Olsalazine is an anti-inflammatory agent that blocks the production of cyclooxygenase (COX)-derived products of arachidonic acid metabolism. It works to alleviate inflammation in ulcerative colitis.
Olsalazine was shown to decrease water and sodium absorption. While olsalazine is not expected to affect the motility and transit time of small intestines, it decreased small bowel transit time at high doses ranging from 60 to 120 mg/kg. In about 40% of the patients with ulcerative colitis, administration of olsalazine 1g daily was associated with a decrease in whole gut transit time.
Veterinary Drugs and Treatments
Olsalazine is used for treatment of dogs with chronic colitis that either cannot tolerate the adverse effects associated with sulfasalazine or the response to sulfasalazine has been ineffective.
Metabolism
Olsalazine is cleaved by colonic bacteria to release its active ingredient, mesalamine. Mesalamine can undergo acetylation to form N-acetyl-5-aminosalicylic acid (N-acetyl-5-ASA, Ac-5-ASA) by the colonic epithelium; however, the extent of acetylation depends on transit time. Approximately 0.1% of an oral dose of olsalazine is metabolized in the liver to olsalazine-O-sulfate (olsalazine-S).
Properties of OLSALAZINE
| Melting point: | >300 °C(Solv: acetic acid (64-19-7); water (7732-18-5)) |
| Boiling point: | 443.29°C (rough estimate) |
| Density | 1.3578 (rough estimate) |
| refractive index | 1.6500 (estimate) |
| pka | 2.40±0.10(Predicted) |
| form | Solid |
| color | Light yellow to yellow |
| Water Solubility | 11.49ug/L(25 ºC) |
| CAS DataBase Reference | 15722-48-2(CAS DataBase Reference) |
Safety information for OLSALAZINE
Computed Descriptors for OLSALAZINE
Abamectin manufacturer
Varanous Labs Pvt Ltd
Nivika Chemo Pharma Pvt Ltd
New Products
4-AMINO-TETRAHYDRO-PYRAN-4-CARBOXYLIC ACID HCL 4-(Dimethylamino)tetrahydro-2H-pyran-4-carbonitrile 4-Aminotetrahydropyran-4-carbonitrile Hydrochloride (R)-3-Aminobutanenitrile Hydrochloride 3-((Dimethylamino)methyl)-5-methylhexan-2-one oxalate 1,4-Dioxa-8-azaspiro[4.5]decane 5-Bromo-2-nitropyridine Nimesulide BP Aceclofenac IP/BP/EP Diclofenac Sodium IP/BP/EP/USP Mefenamic Acid IP/BP/EP/USP Ornidazole IP Diclofenac Potassium THOMAIND PAPER PH 2.0 TO 4.5 1 BOX BUFFER CAPSULE PH 9.2 - 10 CAP SODIUM CHLORIDE 0.1N CVS ALLOXAN MONOHYDRATE 98% PLATINUM 0.5% ON 3 MM ALUMINA PELLETS (TYPE 73) LITHIUM AAS SOLUTION 2-Bromo-1-(bromomethyl)-3-chloro-5-nitrobenzene 2-Bromo-3-nitroaniline N-(3-Hydroxypropyl)-N-methylacetamide 3-Bromo-6-chloropyridazine 4-ethyl-3-nitrobenzoic acidRelated products of tetrahydrofuran



You may like
-
 15722-48-2 Olsalazine 98%View Details
15722-48-2 Olsalazine 98%View Details
15722-48-2 -
 Olsalazine 99%View Details
Olsalazine 99%View Details -
 1823368-42-8 98%View Details
1823368-42-8 98%View Details
1823368-42-8 -
 2-(3-(tert-butyl)phenoxy)-2-methylpropanoic acid 1307449-08-6 98%View Details
2-(3-(tert-butyl)phenoxy)-2-methylpropanoic acid 1307449-08-6 98%View Details
1307449-08-6 -
 Ethyl 3-(furan-2-yl)-3-hydroxypropanoate 25408-95-1 98%View Details
Ethyl 3-(furan-2-yl)-3-hydroxypropanoate 25408-95-1 98%View Details
25408-95-1 -
 2-Chloro-5-fluoro-1-methoxy-3-methylbenzene 98%View Details
2-Chloro-5-fluoro-1-methoxy-3-methylbenzene 98%View Details
1805639-70-6 -
 1784294-80-9 98%View Details
1784294-80-9 98%View Details
1784294-80-9 -
 Lithium ClavulanateView Details
Lithium ClavulanateView Details
61177-44-4