6-Mercaptopurine
- CAS NO.:50-44-2
- Empirical Formula: C5H4N4S
- Molecular Weight: 152.18
- MDL number: MFCD00599524
- EINECS: 200-037-4
- SAFETY DATA SHEET (SDS)
- Update Date: 2025-02-20 21:50:18
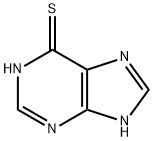
What is 6-Mercaptopurine ?
Absorption
Clinical studies have shown that the absorption of an oral dose of mercaptopurine in humans is incomplete and variable, averaging approximately 50% of the administered dose. The factors influencing absorption are unknown.
Toxicity
Signs and symptoms of overdosage may be immediate such as anorexia, nausea, vomiting, and diarrhea; or delayed such as myelosuppression, liver dysfunction, and gastroenteritis. The oral LD50 of mercaptopurine was determined to be 480 mg/kg in the mouse and 425 mg/kg in the rat.
Chemical properties
Yellowish-Greenish Solid
Originator
Purinethol,Sandoz,France,1950
The Uses of 6-Mercaptopurine
Has a cytotoxicity effect
The Uses of 6-Mercaptopurine
6-Mercaptopurine (6-MP) is a antineoplastic, immonusupressant; inhibits purine nucleotide synthesis and metabolism. 6-MP have been used for several decades in the treatment of leukemia and certain other neoplastic diseases. 6-Mercaptopurine is used for maintenance therapy of acute lymphocytic and acute myelogenous leukemia.
Indications
For remission induction and maintenance therapy of acute lymphatic leukemia.
Background
An antimetabolite antineoplastic agent with immunosuppressant properties. It interferes with nucleic acid synthesis by inhibiting purine metabolism and is used, usually in combination with other drugs, in the treatment of or in remission maintenance programs for leukemia.
What are the applications of Application
6-Mercaptopurine is an inhibitor of de novo purine synthesis
Definition
A sulfurcontaining purine base not found in animal nucleoproteins.
Manufacturing Process
7.5 g of 4-amino-6-chloro-5-nitropyrimidine was suspended in 200 ml of 1 N potassium hydrosulfide and heated on the steam bath for 2 hours while passing hydrogen sulfide through the reaction mixture. The reaction mixture was allowed to cool slowly, acidified with 10 N sulfuric acid and chilled. The precipitate consisted of 4,5-diamino-6-mercaptopyrimidine and sulfur. It was boiled with 300 ml of water, filtered hot and then chilled. The product precipitated as pale yellow needles (4.2 g); an additional 0.95 g was obtained by concentration of the mother liquors to 100 ml.
A mixture of 2 g of 4,5-diamino-6-mercaptopyrimidine and 10 ml of 98% formic acid was heated at 70°C for two hours and then evaporated to dryness on the steam bath to give as a residue, 7-amino-thiazolo (5,4-d) pyrimidine.
To 820 mg of 7-amino-thiazolo[5,4-d]pyrimidine was added 2.5 cc of 2 N sodium hydroxide. The water was removed under reduced pressure. The sodium salt was then heated at 240°C for one hour, during which time it melted, gave off water and resolidified. The sodium salt of 6-mercaptopurine was dissolved in 15 ml of water and acidified to pH 5 with acetic acid. Yellow crystals of 6-mercaptopurine hydrate precipitated, according to US Patent 2,933,498.
brand name
Purinethol (Teva).
Therapeutic Function
Cancer chemotherapy
Hazard
Questionable carcinogen.
Pharmacokinetics
Mercaptopurine is one of a large series of purine analogues which interfere with nucleic acid biosynthesis and has been found active against human leukemias. It is an analogue of the purine bases adenine and hypoxanthine. It is not known exactly which of any one or more of the biochemical effects of mercaptopurine and its metabolites are directly or predominantly responsible for cell death.
Safety Profile
Poison by ingestion, intraperitoneal, subcutaneous, parenteral, and intravenous routes. Human systemic effects by ingestion: dermatitis. Experimental teratogenic and reproductive effects. Questionable human carcinogen producing Hodgkin's disease and leukemia. Human mutation data reported. When heated to decomposition it emits very toxic fumes of SOx and NOx. See also MERCAPTANS.
Veterinary Drugs and Treatments
Veterinary uses of mercaptopurine include adjunctive therapy of lymphosarcoma, acute leukemias, and severe rheumatoid arthritis. It may have potential benefit in treating other autoimmune conditions (e.g., unresponsive ulcerative colitis) as well.
Metabolism
Hepatic. Degradation primarily by xanthine oxidase. The catabolism of mercaptopurine and its metabolites is complex. In humans, after oral administration of 35S-6-mercaptopurine, urine contains intact mercaptopurine, thiouric acid (formed by direct oxidation by xanthine oxidase, probably via 6-mercapto-8-hydroxypurine), and a number of 6-methylated thiopurines. The methylthiopurines yield appreciable amounts of inorganic sulfate.
Properties of 6-Mercaptopurine
| Melting point: | 241-244°C |
| Boiling point: | 498.6±37.0 °C(Predicted) |
| Density | 1.463 (estimate) |
| refractive index | 1.5605 (estimate) |
| storage temp. | Inert atmosphere,2-8°C |
| solubility | DMSO : 35.71 mg/mL (234.66 mM; Need ultrasonic) |
| pka | 7.77, 11.17(at 25℃) |
| form | solid |
| color | Light yellow to yellow |
| Water Solubility | 124mg/L(25 ºC) |
| CAS DataBase Reference | 50-44-2(CAS DataBase Reference) |
| IARC | 3 (Vol. 26, Sup 7) 1987 |
| EPA Substance Registry System | Mercaptopurine (50-44-2) |
Safety information for 6-Mercaptopurine
| Signal word | Warning |
| Pictogram(s) |
 Exclamation Mark Irritant GHS07 |
| GHS Hazard Statements |
H302:Acute toxicity,oral H315:Skin corrosion/irritation H319:Serious eye damage/eye irritation H335:Specific target organ toxicity, single exposure;Respiratory tract irritation |
| Precautionary Statement Codes |
P261:Avoid breathing dust/fume/gas/mist/vapours/spray. P280:Wear protective gloves/protective clothing/eye protection/face protection. P301+P312:IF SWALLOWED: call a POISON CENTER or doctor/physician IF you feel unwell. P302+P352:IF ON SKIN: wash with plenty of soap and water. P305+P351+P338:IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. Continuerinsing. |
Computed Descriptors for 6-Mercaptopurine
6-Mercaptopurine manufacturer
Godavari Drugs Limited
Clickchem Research LLP
New Products
1-Boc-4-cyanopiperidine tert-Butyl carbazate 1-(TERT-BUTOXYCARBONYL)-2-PYRROLIDINONE TETRABUTYLAMMONIUM CYANIDE TETRAHYDRO-2H-PYRAN-3-OL 3-Pyridineacrylic acid Nickel(II) perchlorate hexahydrate, 98% 4-Bromophenylacetonitrile, 95% 3-Bromo-4-fluoroaniline, 97% Sodium tetraborate decahydrate, 98% Palladium(II) acetate, trimer, Pd 99% 4-Bromo-2-chlorotoluene, 97% Tadalafil Clopidogrel bisulfate Sitagliptin Phosphate Monohydrate Cabergoline Fexofinadine HCl Etoricoxib 4-Amino Acetophenone 2-Chloro Acetophenone Amlodipine Base 2,3,5-Triiodobenzoic Acid Pyrrolidine Diiodo PentoxideRelated products of tetrahydrofuran
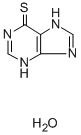

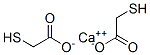

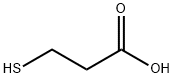
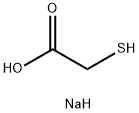
You may like
-
 50-44-2 6-Mercaptopurine 98%View Details
50-44-2 6-Mercaptopurine 98%View Details
50-44-2 -
 50-44-2 98%View Details
50-44-2 98%View Details
50-44-2 -
 6-Mercaptopurine 98%View Details
6-Mercaptopurine 98%View Details
50-44-2 -
 50-44-2 98%View Details
50-44-2 98%View Details
50-44-2 -
 6-Mercaptopurine 98%View Details
6-Mercaptopurine 98%View Details
50-44-2 -
 50-44-2 90 % AboveView Details
50-44-2 90 % AboveView Details
50-44-2 -
 366789-02-8 Riveroxaban 98%View Details
366789-02-8 Riveroxaban 98%View Details
366789-02-8 -
 Carvedilol 98%View Details
Carvedilol 98%View Details
72956-09-3
