Oleoyl Ethanolamide
Synonym(s):oleoylethanolamide;OEA;9Z-octadecenoylethanolamide;N-(cis-9-Octadecenoyl)ethanolamine;N-(Hydroxyethyl)oleamide
- CAS NO.:111-58-0
- Empirical Formula: C20H39NO2
- Molecular Weight: 325.53
- MDL number: MFCD00045972
- EINECS: 203-884-8
- SAFETY DATA SHEET (SDS)
- Update Date: 2025-12-26 16:58:18
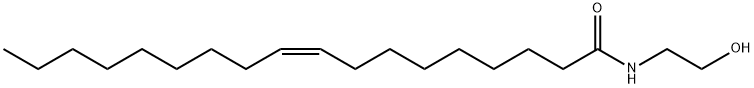
What is Oleoyl Ethanolamide?
Description
Oleoyl ethanolamide (OEA) is an analog of the endocannabinoid AEA found in brain tissue and in chocolate. It is one of the long chain fatty acid ethanolamides that accumulates rapidly in infarcted tissue, but its biosynthesis is reduced in the intestine of rats following food deprivation. OEA is an endogenous, potent agonist for PPARα, exhibiting an EC50 value of 120 nM in a transactivation assay. Systemic administration of OEA suppresses food intake and reduces weight gain in rats (10 mg/kg intraperitoneally) and PPARα wild-
Description
Oleoylethanolamide (OEA) is a lipid that binds the α-type peroxisome proliferator-activated receptor (PPAR-α), which helps the body absorb, store, and use fat. It is produced in the small intestine and regulates feeding and body fat in vertebrates.
Recently, Wiebke K. Fenske and co-workers at Leipzig University discovered a?connection between gastric bypass surgery and OEA. When the researchers performed the surgery on rats, they noticed that afterward the animals preferred low-fat to high-fat foods in their diets. Simultaneously, they observed that the intestinal biosynthesis of OEA increased.
Subsequent investigation revealed that the OEA pathway connects the intestines to the brain via the vagus nerve. When the scientists inhibited PPAR-α or severed the vagus nerve, the rats returned to their high-fat preference. Commenting on this study, other obesity researchers noted that if these results are confirmed in humans, OEA’s importance in the overall diet–obesity–surgery process must be established.
The Uses of Oleoyl Ethanolamide
N-Oleoyl Ethanolamide is an agonist of peroxisome proliferator-activated receptor-α (PPAR-α). N-Oleoyl Ethanolamide generates an intestinal signal that stimulates central dopamine activity establishing a link between caloric-homeostatic and hedonic-homeostatic controllers. Oleoyl Ethanolamide has been implicated as the molecular mechanism associated with gastric bypass success. N-Oleoyl Ethanolamide is a selective GPR55 agonist.
What are the applications of Application
Oleylethanolamide is an activator of Neu/ErbB2 receptors and a mediator of p38 and JNK kinases
Definition
ChEBI: Oleoyl ethanolamide is an N-(long-chain-acyl)ethanolamine that is the ethanolamide of oleic acid. The monounsaturated analogue of the endocannabinoid anandamide. It has a role as a PPARalpha agonist, an EC 3.5.1.23 (ceramidase) inhibitor and a geroprotector. It is a N-(long-chain-acyl)ethanolamine, an endocannabinoid and a N-acylethanolamine 18:1. It is functionally related to an oleic acid.
What are the applications of Application
N-Oleoyl Ethanolamide has been used to study its effects on glucagon-like peptide (GLP)-1RA-mediated anorectic signaling and weight loss.
Biosynthesis
Oleoylethanolamide (OEA) is produced by the small intestine following feeding in two steps. First an N-acyl transferase (NAT) activity joins the free amino terminus of phosphatidylethanolamine (PE) to the oleoyl group (one variety of acyl group) derived from sn-1-oleoyl-phosphatidylcholine, which contains the fatty acid oleic acid at the sn-1 position. This produces an N-acylphosphatidylethanolamine, which is then split (hydrolyzed) by N-acyl phosphatidylethanolamine-specific phospholipase D (NAPE-PLD) into phosphatidic acid and OEA. The biosynthesis of OEA and other bioactive lipid amides is modulated by bile acids.
Biological Functions
N-Oleoyl Ethanolamide is also a predominant NAE species in the injured rat brain, and it has also been found to be the major NAE species in a human brain that has suffered a hemispheric stroke. As early as 1975, N-Oleoyl Ethanolamide was synthesized as an inhibitor of ceramidase, the enzyme that degrades ceramide. Ceramide is involved in the regulation of apoptosis and cell proliferation. Cannabinoid-induced apoptosis in glioma cells is mediated via formation of ceramide. On a tentative basis, it can be suggested that anandamide-induced apoptosis may be aggravated by the presence of N-Oleoyl Ethanolamide because this leads to increased formation of ceramide. There are numerous studies in which N-Oleoyl Ethanolamide has been shown to facilitate the apoptosis-inducing effect of different compounds mediated via increased ceramide levels. However, it has also been reported that N-Oleoyl Ethanolamide decreases ceramide levels in JB6 P+ cells by an unknown mechanism. Recently, N-Oleoyl Ethanolamide has been shown to have a CB1-receptor-independent anorexic effect by inhibiting some intestinal neuronal functions in the rat, and it also causes vasodilation in rat mesenteric arterial segments by an unknown receptor mechanism. Whether these recently discovered biological effects of N-Oleoyl Ethanolamide are of significance for neuroprotection is not known, but it indicates that nonendocannabinoid NAEs are also bioactive molecules with potential for cerebral actions.
General Description
Oleoylethanolamide (OEA) is an endogenous fatty acid ethanolamine. Small intestine cells, adipose tissues, neurons and astrocytes produces OEA.
Biological Activity
Lipid mediator and analog of anandamide (N-(2-Hydroxyethyl)-5Z,8Z,11Z,14Z-eicosatetraenamide ) that is involved in peripheral regulation of feeding. Selective GPR55 agonist (EC 50 values are 0.44, >30 and >30 μ M at GPR55, CB 1 and CB 2 respectively) and PPAR α agonist (EC 50 = 120 nM). Induces satiety through activation of PPAR α and is also a ceramidase inhibitor. Also endogenous agonist at the GPR119 receptor.
Biochem/physiol Actions
Oleoylethanolamide (OEA) is a lipid mediator, which helps to control various biological functions, like food intake. It modulates the gene expression in the small intestine. OEA has the ability to initiate several receptors, such as cannabinoid receptor type 1 (CB1), peroxisome proliferator-activated receptors (PPARs) and G protein-coupled receptor 119 (GPR119).
Enzyme inhibitor
This endocannabinoid-related metabolite (N-Oleoyl Ethanolamide; FW = 325.54 g/mol; CAS 111- 58-0; Symbol: NOE and OEA; Soluble in Ethanol, Chloroform, or Methanol) is widely employed to inhibit acid and neutral ceramidases (IC50 ~ 500 μM) that cleave fatty acids from ceramide, producing sphingosine (SPH), which is then enzymatically phosphorylated to form the receptorsensed metabolite, sphingosine-1-phosphate, or S1P. NOE also inhibits the glucosylation of naturally occurring ceramides. In CHP-100 neuroepithelioma cells treated with N-hexanoylsphingosine (C6-Cer; 30 μM), NOE affected only marginally short-chain glucocerebroside accumulation, but markedly decreased accumulation of glucocerebrosides originating from glucosylation of a long-chain ceramide (Lc-Cer) produced upon C6-Cer treatment. NOE also inhibits fatty acid hydrolase (or FAAH), an integral membrane hydrolase possessing an unusual catalytic triad Ser-Ser-Lys.
N-Oleoyl Ethanolamide is also an effective inhibitor of mitochondrial swelling, but does not inhibit phospholipase A2 or ruthenium red-induced Ca2+ release. Moreover, among naturally occurring Nacylethanolamines, only NOE (at 10 μM) inhibits the accumulation of Narachidonoylethanolamine (or Anandamide, AEA), a putative endogenous ligand of the cannabinoid receptor, into cerebellar granule cells occurs by means of facilitated diffusion.
Storage
Store at +4°C
References
1) Lo Verme et al. (2005), Regulation of food intake by oleoylethanolamide; Cell Mol. Life Sci. 62 708
2) Magotti et al. (2015), Structure of human N-acylphosphatidylethanolamine-hydrolyzing phospholipase D: regulation of fatty acid ethanolamide biosynthesis by bile acids; Structure 23 598
3) Overton et al. (2006), Deorphanization of a G protein-coupled receptor for oleoylethanolamide and its use in the discovery of small-molecules hypophagic agents; Cell Metab. 3 167
4) Brown (2007), Novel cannabinoid receptors; Br. J. Pharmacol. 152 567
5) Nielson et al. (2004), Food intake is inhibited by oral oleoylethanolamide; J. Lipid Res. 45 1027
6) Folick et al. (2015), Aging. Lysosomal signaling molecules regulate longevity in Caenorhabditis elegans; Science 347 83
Properties of Oleoyl Ethanolamide
| Melting point: | 63-64 °C |
| Boiling point: | 496.4±38.0 °C(Predicted) |
| Density | 0.915±0.06 g/cm3(Predicted) |
| storage temp. | -20°C |
| solubility | Soluble in DMSO (up to 25 mg/ml) or in Ethanol (up to 35 mg/ml) |
| form | White solid |
| pka | 14.49±0.10(Predicted) |
| color | White |
| Stability: | Stable for 2 years from date of purchase as supplied. Solutions in DMSO or ethanol may be stored at -20°C for up to 1 month. |
| InChI | InChI=1S/C20H39NO2/c1-2-3-4-5-6-7-8-9-10-11-12-13-14-15-16-17-20(23)21-18-19-22/h9-10,22H,2-8,11-19H2,1H3,(H,21,23)/b10-9- |
| CAS DataBase Reference | 111-58-0(CAS DataBase Reference) |
| EPA Substance Registry System | 9-Octadecenamide, N-(2-hydroxyethyl)-, (9Z)- (111-58-0) |
Safety information for Oleoyl Ethanolamide
Computed Descriptors for Oleoyl Ethanolamide
| InChIKey | BOWVQLFMWHZBEF-KTKRTIGZSA-N |
| SMILES | C(NCCO)(=O)CCCCCCC/C=C\CCCCCCCC |
Oleoyl Ethanolamide manufacturer
Indexim International
New Products
4,4-Difluoropiperidine hydrochloride tert-butyl 9-methoxy-3-azaspiro[5.5]undecane-3-carboxylate Indole Methyl Resin N-Isopropylurea N,N-Dicyclohexylcarbodiimide(DCC) MELDRUMS ACID 5-METHYLISOXAZOLE-4-CARBOXYLIC ACID Magnessium Bis glycinate Zinc ascorbate 1-bromo-2-butyne 2-acetamidophenol 9(10H)-anthracenone Erythrosin B, 4-Piperidinopiperidine 2-((4-morpholinophenylamino) (methylthio) methylene) malononitrile 2,4-dihydroxybenzaldehyde 3-(4-morpholinophenylamino)-5-amino-1H-pyrazole-4-carbonitrile Methyl 2-methylquinoline-6-carboxylate 2,6-dichloro-4-nitropyridine 4-Bromo-2-chlorobenzonitrile 2-(benzylamino)acetic acid hydrochloride 4-(tert-Butoxycarbonylamino)but- 2-ynoic acid 3,4-dihydro-2H-benzo[b][1,4]dioxepine 1-Phenyl-1-cycloprppanecarboxylicacidRelated products of tetrahydrofuran
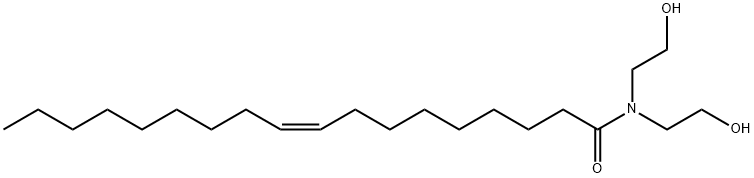
![disodium (Z)-[2-[(1-oxooctadec-9-enyl)amino]ethyl] 2-sulphonatosuccinate](https://img.chemicalbook.in/CAS/GIF/68479-64-1.gif)
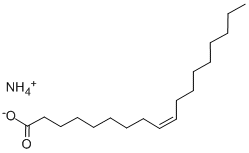
![disodium 1-[1-methyl-2-[(1-oxooctadec-9-enyl)amino]ethyl] 2-sulphonatosuccinate](https://img.chemicalbook.in/CAS/GIF/43154-85-4.gif)

You may like
-
 N-Oleoylethanolamine 97% CAS 111-58-0View Details
N-Oleoylethanolamine 97% CAS 111-58-0View Details
111-58-0 -
 N-Oleoylethanolamine 95.00% CAS 111-58-0View Details
N-Oleoylethanolamine 95.00% CAS 111-58-0View Details
111-58-0 -
 N-Oleoylethanolamine CAS 111-58-0View Details
N-Oleoylethanolamine CAS 111-58-0View Details
111-58-0 -
 Oleoylethanolamide (OEA), CAS No: 111-58-0, N-Oleoylethanolamide, For Laboratory, PowderView Details
Oleoylethanolamide (OEA), CAS No: 111-58-0, N-Oleoylethanolamide, For Laboratory, PowderView Details
111-58-0 -
 3-(4-amino-1-oxoisoindolin-2-yl)-1-methylpiperidine-2,6-dione 98%View Details
3-(4-amino-1-oxoisoindolin-2-yl)-1-methylpiperidine-2,6-dione 98%View Details -
 20677-73-0 (2,2-diethoxyethyl)methylamine 98%View Details
20677-73-0 (2,2-diethoxyethyl)methylamine 98%View Details
20677-73-0 -
 3-(4-(hydroxyamino)-1-oxoisoindolin-2-yl)piperidine-2,6-dione 98%View Details
3-(4-(hydroxyamino)-1-oxoisoindolin-2-yl)piperidine-2,6-dione 98%View Details -
 57381-49-4 2-bromo-4-chlorobenzonitrile 98%View Details
57381-49-4 2-bromo-4-chlorobenzonitrile 98%View Details
57381-49-4
