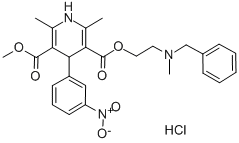
Nicardipine hydrochloride
Synonym(s):1,4-Dihydro-2,6-dimethyl-4-(3-nitrophenyl)-3,5-pyridinedicarboxylic acid methyl 2-[methyl(phenylmethyl)amino]ethyl ester hydrochloride;Nicardipine hydrochloride;YC-93
- CAS NO.:54527-84-3
- Empirical Formula: C26H30ClN3O6
- Molecular Weight: 515.99
- MDL number: MFCD00057327
- EINECS: 259-198-4
- SAFETY DATA SHEET (SDS)
- Update Date: 2025-01-27 09:38:02
What is Nicardipine hydrochloride?
Description
Nicardipine is a dihydropyridine L-type calcium channel antagonist that displays antihypertensive and antianginal activity. It is reported to inhibit adenosine A1, A2A, and A3 receptors with Ki values of 19.6, 63.8, and 3.25 μM, respectively, and can inhibit cytochrome P450 3A4 catalytic activity with an IC50 value of 0.148 μM. Additionally, nicardipine has been shown to activate transient receptor potential A1 channels, producing an increase in Ca2+ (EC50 = 0.5 μM).
Chemical properties
Yellow Solid
The Uses of Nicardipine hydrochloride
Dihydropyridine calcium channel blocker. Antianginal; antihypertensive
The Uses of Nicardipine hydrochloride
anesthetic (topical)
The Uses of Nicardipine hydrochloride
Nicardipine is a dihydropyridine L-type calcium channel antagonist that displays antihypertensive and antianginal activity. It is reported to inhibit adenosine A1, A2A, and A3 receptors with Ki values of 19.6, 63.8, and 3.25 μM, respectively, and can inhibit cytochrome P450 3A4 catalytic activity with an IC50 value of 0.148 μM. Additionally, nicardipine has been shown to activate transient receptor potential A1 channels, producing an increase in Ca2+ (EC50 = 0.5 μM).
What are the applications of Application
Nicardipine hydrochloride is a potent calcium channel protein inhibitor
Definition
ChEBI: Nicardipine hydrochloride is a dihydropyridine. It has a role as a geroprotector.
brand name
Cardene(PDL Biopharma); Cardene (Roche).
General Description
Nicardipine hydrochloride,1,4-dihydro-2,6-dimethyl-4-(3-nitrophenyl)-3,5-pyridinedicarboxylicacid methyl 2-[methyl(phenylmethyl)amino]ethylester hydrochloride (Cardene), is a more potent vasodilator ofthe systemic, coronary, cerebral, and renal vasculature andhas been used in the treatment of mild, moderate, and severehypertension. The drug is also used in the management of stableangina.
Biochem/physiol Actions
Blocks L-type voltage-dependent calcium channels; antihypertensive.
Clinical Use
Calcium-channel blocker:
Prophylaxis and treatment of angina
Mild to moderate hypertension
Acute life-threatening hypertension and post operative hypertension (IV)
Safety Profile
Poison by ingestion, intravenous, and intraperitoneal routes. Moderately toxic by subcutaneous route. Human systemic effects: decreased urine volume or anuria. Experimental reproductive effects. When heated to decomposition it emits toxic fumes of NOx an
Drug interactions
Potentially hazardous interactions with other drugs
Aminophylline: possibly increases aminophylline
concentration.
Anaesthetics: enhanced hypotensive effect.
Antibacterials: metabolism possibly accelerated
by rifampicin; metabolism possibly inhibited by
clarithromycin, erythromycin and telithromycin.
Antidepressants: enhanced hypotensive effect with
MAOIs.
Antiepileptics: effect reduced by carbamazepine,
barbiturates, phenytoin and primidone.
Antifungals: metabolism possibly inhibited by
itraconazole and ketoconazole; negative inotropic
effect possibly increased with itraconazole.
Antihypertensives: enhanced hypotensive effect,
increased risk of first dose hypotensive effect of post synaptic alpha-blockers.
Antivirals: concentration possibly increased by
ritonavir; use telaprevir with caution.
Cardiac glycosides: digoxin concentration increased.
Ciclosporin: concentration of ciclosporin increased
Grapefruit juice: concentration increased - avoid.
Tacrolimus: may increase tacrolimus levels.
Theophylline: possibly increased theophylline
concentration.
Metabolism
Nicardipine is subject to saturable first-pass metabolism. It is extensively metabolised in the liver and excreted in the urine and faeces, mainly as inactive metabolites.
References
1) Merck 14 6495 2) Hulubei et al. (2012), 4-Isoxazolyl-1,4-dihydropyridines exhibit binding at the multidrug-resistance transporter; Bioorg. Med. Chem., 20 6620
Properties of Nicardipine hydrochloride
| Melting point: | 176-1780C |
| storage temp. | 2-8°C |
| solubility | DMSO: ~1 mg/mL |
| form | powder |
| color | yellow |
| Merck | 14,6495 |
| Stability: | Stable for 1 year from date of purchase as supplied. Solutions in distilled water or ethanol may be stored at -20°C for up to 1 month. |
| CAS DataBase Reference | 54527-84-3(CAS DataBase Reference) |
Safety information for Nicardipine hydrochloride
| Signal word | Danger |
| Pictogram(s) |
 Skull and Crossbones Acute Toxicity GHS06 |
| Precautionary Statement Codes |
P280:Wear protective gloves/protective clothing/eye protection/face protection. |
Computed Descriptors for Nicardipine hydrochloride
| InChIKey | AIKVCUNQWYTVTO-UHFFFAOYSA-N |
New Products
3-Iodophenylacetic acid 3-Pyridineacetonitrile, α-hydroxy- 2-Propanamine, 1-chloro-, hydrochloride (9CI) 3-(hexyloxy)-4-(pyridin-3-yl)-1,2,5-thiadiazole 2-Hexyn-1-ol Dibenzo-18-crown-6 Nickel(II) perchlorate hexahydrate, 98% 4-Bromophenylacetonitrile, 95% 3-Bromo-4-fluoroaniline, 97% Sodium tetraborate decahydrate, 98% Palladium(II) acetate, trimer, Pd 99% 4-Bromo-2-chlorotoluene, 97% N N Dimethylformamide Dimethyl Acetal (Dmf Dma) 2,3-Dichloro Benzoyl Cyanide [Side Chain] Bis(2-Chloroethyl) Amine Hydrochloride L-Glutamic Acid Diethyl Ester Hydrochloride 5-(Difluoromethoxy)-2-Mercaptobenzimidazole 1-Ethyl-3-(3-Dimethylaminopropyl)-Carbodiimide Hydrochloride [EDC Hcl] 1,4-Napthoquinone Bromoiodomethane Sodium Bicarbonate Methylene Dichloride (MDC) Ethyl Acetate Indole-3-Carbinol (I3C)Related products of tetrahydrofuran





You may like
-
 Nicardipine Hydrochloride CAS 54527-84-3View Details
Nicardipine Hydrochloride CAS 54527-84-3View Details
54527-84-3 -
 Nicardipine HCl 98% CAS 54527-84-3View Details
Nicardipine HCl 98% CAS 54527-84-3View Details
54527-84-3 -
 Nicardipine hydrochloride CAS 54527-84-3View Details
Nicardipine hydrochloride CAS 54527-84-3View Details
54527-84-3 -
 Nicardipine hydrochloride CAS 54527-84-3View Details
Nicardipine hydrochloride CAS 54527-84-3View Details
54527-84-3 -
 Nicardipine hydrochloride CAS 54527-84-3View Details
Nicardipine hydrochloride CAS 54527-84-3View Details
54527-84-3 -
 54527-84-3 98%View Details
54527-84-3 98%View Details
54527-84-3 -
 Cyclohexane, (2-propynyloxy)- 67967-07-1 98+View Details
Cyclohexane, (2-propynyloxy)- 67967-07-1 98+View Details
67967-07-1 -
 132945-75-6 (S)-1-Boc-3-methanesulfonyloxy-pyrrolidine 98+View Details
132945-75-6 (S)-1-Boc-3-methanesulfonyloxy-pyrrolidine 98+View Details
132945-75-6
