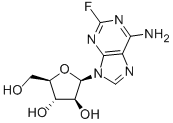
Nelarabine
- CAS NO.:121032-29-9
- Empirical Formula: C11H15N5O5
- Molecular Weight: 297.27
- MDL number: MFCD00871078
- EINECS: 642-916-9
- SAFETY DATA SHEET (SDS)
- Update Date: 2025-01-27 09:38:02
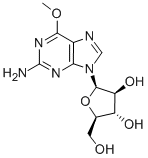
What is Nelarabine?
Absorption
Following intravenous administration of nelarabine to adult patients with refractory leukemia or lymphoma, plasma ara-G Cmax values generally occurred at the end of the nelarabine infusion and were generally higher than nelarabine Cmax values, suggesting rapid and extensive conversion of nelarabine to ara-G. Mean plasma nelarabine and ara-G Cmax values were 5.0 ± 3.0 mcg/mL and 31.4 ± 5.6 mcg/mL, respectively, after a 1,500 mg/m2 nelarabine dose infused over 2 hours in adult patients. The area under the concentration-time curve (AUC) of ara-G is 37 times higher than that for nelarabine on Day 1 after nelarabine IV infusion of 1,500 mg/m2 dose (162 ± 49 mcg.h/mL versus 4.4 ± 2.2 mcg.h/mL, respectively). Comparable Cmax and AUC values were obtained for nelarabine between Days 1 and 5 at the nelarabine adult dosage of 1,500 mg/m2, indicating that nelarabine does not accumulate after multiple dosing. There are not enough ara-G data to make a comparison between Day 1 and Day 5. After a nelarabine adult dose of 1,500 mg/m2, intracellular Cmax for ara-GTP appeared within 3 to 25 hours on Day 1. Exposure (AUC) to intracellular ara-GTP was 532 times higher than that for nelarabine and 14 times higher than that for ara-G (2,339 ± 2,628 mcg.h/mL versus 4.4 ± 2.2 mcg.h/mL and 162 ± 49 mcg.h/mL, respectively).
Toxicity
A single IV dose of 4,800 mg/m^2 was lethal in monkeys, and was associated with CNS signs including reduced/shallow respiration, reduced reflexes, and flaccid muscle tone. It is anticipated that overdosage would result in severe neurotoxicity (possibly including paralysis, coma), myelosuppression, and potentially death.
Description
Nelarabine is a new member of the purine nucleoside antimetabolite class of drugs. It was launched as an intravenous infusion for treating relapsed or refractory T-cell acute lymphoblastic leukemia (T-ALL) and T-cell lymphoblastic lymphoma (T-LBL) after at least two prior chemotherapy regimens. Nelarabine is a pro-drug of 9-b-D-arabinofuranosylguanine (ara-G), a deoxyguanosine derivative with a high level of T-cell selective cytotoxicity. Although Ara-G has been known since the 1960s, it has not been used in clinical studies due to its poor solubility. Nelarabine is the O-methyl derivative of ara-G with approximately 10 times greater aqueous solubility. It is demethylated in vivo by adenosine deaminase to produce ara-G, which is subsequently converted to the active 5’-triphosphate, ara-GTP. Accumulation of ara-GTP in leukemic blasts allows for incorporation into DNA, leading to inhibition of DNA synthesis and cell death.
Originator
Glaxo Wellcome (US)
The Uses of Nelarabine
A chemotherapy drug used in the treatment of relapsed/refractory T-cell acute lymphoblastic leukemia (T-ALL) and T-cell lymphoblastic lymphoma (T-LBL).
Background
Nelarabine is an antineoplastic agent that is typically used to treat acute T-cell lymphoblastic leukemia, particularly T-cell acute lymphoblastic leukemia (T-ALL) and T-cell lymphoblastic lymphoma (T-LBL), in both adult and pediatric patients whose disease has not responded to or has relapsed following at least two chemotherapy regimens. T-cell acute lymphoblastic leukemia and lymphoma are relatively rare T-cells malignancy, with only 20 to 25% of patients diagnosed with acute lymphoblastic leukemia and 1.7% of patients diagnosed with non-Hodgkin's lymphoma having this T-cells variation of the disease. Due to the rarity of these T-cell malignancies, nelarabine was first granted orphan drug status and a fast-track designation by the FDA to address the unmet therapeutic needs of these cancers.
Nelarabine is a purine nucleoside analog converted to its corresponding arabinosylguanine nucleotide triphosphate (araGTP), resulting in the inhibition of DNA synthesis and cytotoxicity. Nelarabine preferentially accumulates in T-cells since T-cells have a higher expression of enzymes that convert nelarabine to the active purine analog form, making them effective against T-cells malignancies. Results from 2 phase 2 studies on adult and pediatric T-ALL/T-LBL indicated that nelarabine can yield a 13% complete response (CR) rate in pediatric patients and 18% in adult patients, albeit with serious hematological and neurological adverse events.
Nelarabine was first granted accelerated approval by the FDA on October 28, 2005, and was manufactured under the trademark name ARRANON by GlaxoSmithKline. Subsequently, nelarabine was also approved by both Health Canada and European Medicines Agency in 2007 under the trademark name ATRIANCE.
Indications
ARRANON is indicated for the treatment of T-cell acute lymphoblastic leukemia (T-ALL) and T-cell lymphoblastic lymphoma (T-LBL) in adult and pediatric patients age 1 year and older whose disease has not responded to or has relapsed following treatment with at least two chemotherapy regimens.
Definition
ChEBI: Nelarabine is a purine nucleoside in which O-methylguanine is attached to arabinofuranose via a beta-N(9)-glycosidic bond. Inhibits DNA synthesis and causes cell death; a prodrug of 9-beta-D-arabinofuranosylguanine (ara-G). It has a role as an antineoplastic agent, a DNA synthesis inhibitor and a prodrug. It is a purine nucleoside, a beta-D-arabinoside and a monosaccharide derivative. It is functionally related to a guanine and a 9-beta-D-arabinofuranosylguanine.
brand name
Arranon (SmithKline Beecham).
Pharmacokinetics
Nelarabine is a prodrug of the cytotoxic deoxyguanosine analogue 9-?-D-arabinofuranosylguanine (ara-G). Nelarabine is demethylated by adenosine deaminase (ADA) to ara-G. Ara-G is then transported into cells, where it undergoes three phosphorylation steps, resulting in the formation of ara-G triphosphate (ara-GTP). In the first phosphorylation step, ara-G is converted to ara-G monophosphate (ara-GMP). Ara-GMP is then monophosphorylated by deoxyguanosine kinase and deoxycytidine kinase to ara-G diphosphate, and then subsequently to the active ara-G triphosphate (ara-GTP). Ara-GTP is the one that exerts the pharmacological effect. Pre-clinical studies have demonstrated that targeted T-cells possess marked sensitivity to the agent. Since T lymphoblasts have a higher expression of deoxycytidine kinase, ara-G preferentially accumulates in T cells over B cells, thus showing higher toxicity to T lymphoblasts.
Clinical Use
Antineoplastic agent:
T-cell acute lymphoblastic leukaemia (T-ALL)
T-cell lymphoblastic lymphoma (T-LBL)
Synthesis
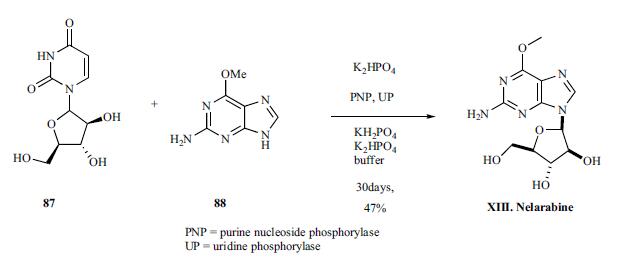
The drug was synthesized by enzymatic coupling of arabinosyluracil 87, prepared according to literature and 2-amino- 6-methoxy purine 88 using purine nucleoside phosphorylase (PNP) and uridine phosphorylase (UP) in phosphate buffer for 30 days to give the nelarabine (XIII) in 48% yield.
Metabolism
The principal route of metabolism for nelarabine is O-demethylation by adenosine deaminase to form ara-G, which undergoes hydrolysis to form guanine. In addition, some nelarabine is hydrolyzed to form methylguanine, which is O-demethylated to form guanine. Guanine is N-deaminated to form xanthine, which is further oxidized to yield uric acid. Ring opening of uric acid followed by further oxidation results in the formation of allantoin.
Ring opening of uric acid followed by further oxidation results in the formation of allantoin.
Metabolism
Nelarabine is a pro-drug of the deoxyguanosine analogue ara-G. Extensive metabolism by O-demethylation by adenosine deaminase to form ara-G, which undergoes hydrolysis to form guanine. In addition, some nelarabine is hydrolysed to form methylguanine, which is O-demethylated to form guanine. Guanine is N-deaminated to form xanthine, which is further oxidised to yield uric acid. Nelarabine and ara-G are partially eliminated by the kidneys.
storage
Store at +4°C
References
[1] cohen mh, johnson jr, massie t, et al. approval summary: nelarabine for the treatment of t-cell lymphoblastic leukemia/lymphoma. clin cancer res. september 2006. 18:5329–35.
[2] yesid alvarado, mary alma welch, ronan swords, john bruzzi, ellen schlette, francis j. giles. nelarabine activity in acute biphenotypic leukemia. leukemia research. november 2007. 31(11): 1600-1603.
Properties of Nelarabine
| Melting point: | 209-217° |
| Boiling point: | 721.0±70.0 °C(Predicted) |
| alpha | D20 +55.9° (c = 0.27 in DMF) |
| Density | 1.98±0.1 g/cm3(Predicted) |
| storage temp. | 2-8°C(protect from light) |
| solubility | DMSO (Slightly), Methanol (Slightly, Heated), Water (Slightly, Sonicated) |
| form | Solid |
| pka | 13.07±0.70(Predicted) |
| color | White |
| InChI | InChI=1/C11H15N5O5/c1-20-9-5-8(14-11(12)15-9)16(3-13-5)10-7(19)6(18)4(2-17)21-10/h3-4,6-7,10,17-19H,2H2,1H3,(H2,12,14,15)/t4-,6-,7+,10-/s3 |
| CAS DataBase Reference | 121032-29-9(CAS DataBase Reference) |
Safety information for Nelarabine
| Signal word | Warning |
| Pictogram(s) |
 Exclamation Mark Irritant GHS07 |
| GHS Hazard Statements |
H302:Acute toxicity,oral H315:Skin corrosion/irritation H319:Serious eye damage/eye irritation H335:Specific target organ toxicity, single exposure;Respiratory tract irritation |
| Precautionary Statement Codes |
P261:Avoid breathing dust/fume/gas/mist/vapours/spray. P305+P351+P338:IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. Continuerinsing. |
Computed Descriptors for Nelarabine
| InChIKey | IXOXBSCIXZEQEQ-GCTRTDOSNA-N |
| SMILES | N1(C=NC2C(OC)=NC(N)=NC1=2)[C@H]1[C@@H](O)[C@H](O)[C@@H](CO)O1 |&1:12,13,15,17,r| |
Nelarabine manufacturer
New Products
3-Iodophenylacetic acid 3-Pyridineacetonitrile, α-hydroxy- 2-Propanamine, 1-chloro-, hydrochloride (9CI) 3-(hexyloxy)-4-(pyridin-3-yl)-1,2,5-thiadiazole 2-Hexyn-1-ol Dibenzo-18-crown-6 Nickel(II) perchlorate hexahydrate, 98% 4-Bromophenylacetonitrile, 95% 3-Bromo-4-fluoroaniline, 97% Sodium tetraborate decahydrate, 98% Palladium(II) acetate, trimer, Pd 99% 4-Bromo-2-chlorotoluene, 97% N N Dimethylformamide Dimethyl Acetal (Dmf Dma) 2,3-Dichloro Benzoyl Cyanide [Side Chain] Bis(2-Chloroethyl) Amine Hydrochloride L-Glutamic Acid Diethyl Ester Hydrochloride 5-(Difluoromethoxy)-2-Mercaptobenzimidazole 1-Ethyl-3-(3-Dimethylaminopropyl)-Carbodiimide Hydrochloride [EDC Hcl] 1,4-Napthoquinone Bromoiodomethane Sodium Bicarbonate Methylene Dichloride (MDC) Ethyl Acetate Indole-3-Carbinol (I3C)Related products of tetrahydrofuran

![(2R,3R,4S,5R)-2-(6-aMino-9H-purin-9-yl)-5-((((1r,3S)-3-(2-(5-(tert-butyl)-1H-benzo[d]iMidazol-2-yl)ethyl)cyclobutyl)(isopropyl)aMino)Methyl)tetrahydrofuran-3,4-diol](https://img.chemicalbook.in/CAS/20150408/GIF/1380288-87-8.gif)
You may like
-
 Nelarabine 98% CAS 121032-29-9View Details
Nelarabine 98% CAS 121032-29-9View Details
121032-29-9 -
 Nelarabine 97.00% CAS 121032-29-9View Details
Nelarabine 97.00% CAS 121032-29-9View Details
121032-29-9 -
 Nelarabine CAS 121032-29-9View Details
Nelarabine CAS 121032-29-9View Details
121032-29-9 -
 17604-74-9 3-Pyridineacetonitrile, α-hydroxy- 98+View Details
17604-74-9 3-Pyridineacetonitrile, α-hydroxy- 98+View Details
17604-74-9 -
 Cyclohexane, (2-propynyloxy)- 67967-07-1 98+View Details
Cyclohexane, (2-propynyloxy)- 67967-07-1 98+View Details
67967-07-1 -
 2-Propanamine, 1-chloro-, hydrochloride (9CI) 98+View Details
2-Propanamine, 1-chloro-, hydrochloride (9CI) 98+View Details
5968-21-8 -
 3-Iodophenylacetic acid 1878-69-9 98+View Details
3-Iodophenylacetic acid 1878-69-9 98+View Details
1878-69-9 -
 132945-75-6 (S)-1-Boc-3-methanesulfonyloxy-pyrrolidine 98+View Details
132945-75-6 (S)-1-Boc-3-methanesulfonyloxy-pyrrolidine 98+View Details
132945-75-6
