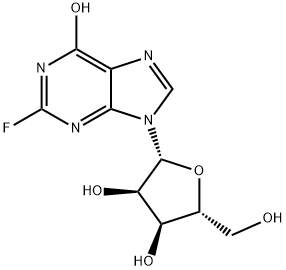
Fludarabine
Synonym(s):9-β-D -Arabinofuranosyl-2-fluoroadenine;F-ara-A;Fludarabine des-phosphate
- CAS NO.:21679-14-1
- Empirical Formula: C10H12FN5O4
- Molecular Weight: 285.23
- MDL number: MFCD00132942
- EINECS: 244-525-5
- SAFETY DATA SHEET (SDS)
- Update Date: 2025-01-27 09:38:02
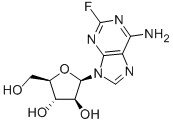
What is Fludarabine?
Absorption
Bioavailability is 55% following oral administration.
Description
Fludarabine (21679-14-1) is a synthetic adenosine analog that inhibits DNA biosynthesis and is a clinically useful antineoplastic agent.1 In cells fludarabine accumulates as its 5’-triphosphate (F-ara-ATP) for which the rate-limiting step in formation is the conversion of fludarabine to its monophosphate.2 F-ara-ATP has multiple mechanisms of action including inhibition of ribonucleotide reductase, DNA polymerase, ligase and primase.2 A frequently used agent in myeloablative conditioning regimens for allogeneic hematopoietic cell transplantation.3 Immunosuppressive effects are mediated via inhibition of TNFa-stimulated production of IL-2 and IFN-g through inactivation of NFkB.4 Antagonist at adenosine A1 receptors.5
Chemical properties
White Solid
Originator
Fludarabine,Union Pharmaceutical
The Uses of Fludarabine
Fludarabine is not one of the most used drugs in pediatric oncology area. It is used in combination with other drugs to treat AML in children, mostly those who are receiving second-line therapy. It is commonly sold as 10 mg film-coated tablets and IV vial containing 50mg.
The Uses of Fludarabine
Fludarabine is used in the treatment of chroniclymphocytic leukaemia. It is a DNA synthesis and methylation inhibitor;A cell permeable agent that interferes with DNA synthesis and repair; also inhibits RNA transcription.
Background
Fludarabine is a chemotherapeutic agent used in the treatment of hematological malignancies. It is commonly marketed under the brand name Fludara.
Indications
For the treatment of adult patients with B-cell chronic lymphocytic leukemia (CLL) who have not responded to or whose disease has progressed during treatment with at least one standard alkylating-agent containing regimen
What are the applications of Application
Fludarabine is a cell permeable agent that interferes with DNA synthesis and repair; also inhibits RNA transcription
Indications
Fludarabine (Fludara) is a fluorinated purine analogue
of the antiviral agent vidarabine.The active metabolite,
2-fluoro-ara-adenosine triphosphate, inhibits various
enzymes involved in DNA synthesis, including DNA
polymerase-α, ribonucleotide reductase, and DNA primase.
Unlike most antimetabolites, it is toxic to nonproliferating
as well as dividing cells, primarily lymphocytes
and lymphoid cancer cells.
The drug is highly active in the treatment of chronic
lymphocytic leukemia, with approximately 40% of patients
achieving remissions after previous therapy with
alkylating agents has failed. Activity is also seen in the
low-grade lymphomas.
The major side effect is myelosuppression, which
contributes to fevers and infections in as many as half of
treated patients. Nausea and vomiting are mild.
Occasional neurotoxicity has been noted at higher
doses, with agitation, confusion, and visual disturbances.
Preparation
Synthesis of fludarabine 1: 2-Fluoroadenine is reacted with 9-β-D-arabinosyl-uracile, taking water as a solvent. The reaction will take place in the presence of Enterobacter aerogenes. Fludarabine so formed is then treated with acetic anhydride to form the acetylderivative.The acetyl derivative is then crystallize to get back the pure Fludarabine.
Synthesis of fludarabine 2: 2,4,5,6-tetraaminopyrimidine and formamide were cyclized together by heating to give 2,6-diaminopurine. acylation with acetic anhydride-pyridine complex gave 2,6-diacetamidopurine. then reacted with 2,3,5-tri-o-benzyl-d-arabinofuranosyl chloride to give compound (i). (i) was deacetylated to give compound (ii). (ii) in fluoroboronic acid-tetrahydrofuran, first nitrosated and then substituted to give compound (ili). in the presence of boron trichloride, the benzyl group was removed to give fludarabine. the yield was 17.5% in terms of 2,3,5-tri-o-benzyl-d-arabinofuranosyl chloride.
Definition
ChEBI: (2R,3S,4S,5R)-2-(6-amino-2-fluoro-9-purinyl)-5-(hydroxymethyl)oxolane-3,4-diol is a purine nucleoside.
Therapeutic Function
Antineoplastic
General Description
The drug is available as the phosphate salt in a 50-mg vialfor IV use. Fludarabine is used to treat chronic lymphocyticleukemia and non-Hodgkin’s lymphoma. The mechanism ofaction involves the triphosphate metabolite and its inhibitionof DNA chain elongation. The 2-fluoro group on the adeninering renders fludarabine resistant to breakdown byadenosine deaminase. The drug is rapidly dephosphorylatedto 2-fluoro-ara-adenosine (F-ara-A) after administration. Fara-A is taken into the cell and subsequently re-phosphorylatedto yield the triphosphate (F-ara-ATP), the active drugspecies. Resistance can occur via decreased expression ofthe activating enzymes and decreased drug transport.Fludarabine is orally bioavailable and is distributed throughoutthe body reaching high levels in liver, kidney, andspleen. The drug is metabolized to F-ara-A, which enterscells via the nucleoside transport system and is rephosphorylatedby deoxycytidine kinase to fludarabine monophosphateand finally fludarabine triphosphate, the activespecies. About 25% of F-ara-A is excreted unchanged inurine. Drug interactions include an increased incidence offatal pulmonary toxicity when fludarabine is used in combinationwith pentostatin. Additionally, fludarabine may potentiate the effects of several other anticancer drugs includingcytarabine, cyclophosphamide, and cisplatin.Toxicities include myelosuppression, immunosuppression,fever, nausea, and vomiting.
Biological Activity
Purine analog that inhibits DNA synthesis. Exhibits antiproliferative activity (IC 50 = 1.54 μ M in RPMI cells) and triggers apoptosis through increasing Bax and decreasing Bid, XIAP and survivin expression. Displays anticancer activity against hematological malignancies in vivo .
Biochem/physiol Actions
Fludarabine (the 5′-phosphate) is a prodrug that is converted to F-ara-A, which enters cells and accumulates primarily as the 5′-triphosphate. F-ara-A interferes with DNA synthesis and repair and induces apoptosis of cancer cells. F-ara-A also strongly inhibits DNA methylation, particularly methylation of cytosine in CpG dinucleotide sequences.
Pharmacokinetics
Fludarabine is a chemotherapy drug used in the treatment of chronic lymphocytic leukemia. It acts at DNA polymerase alpha, ribonucleotide reductase and DNA primase, results in the inhibition of DNA synthesis, and destroys the cancer cells.
Metabolism
Not Available
storage
Desiccate at +4°C
Mode of action
Fludarabine is a fluorinated analogue of adenine that is relatively resistant to deamination by adenosine deaminase. Fludarabine phosphate is a prodrug that is rapidly dephosphorylated to 2-fluoro-ara-A and then phosphorylated intracellularly by deoxycytidine kinase to the active metabolite triphosphate 2-fluoro-ara-ATP. Fludarabine inhibits the DNA synthesis via inhibition of ribonucleotide reductase, DNA polymerase (α, δ, and ε), DNA primase, and DNA ligase. The action mechanism also is by partial inhibition of RNA polymerase II, causing reduction in protein synthesis. It is believed that effects on DNA, RNA, and protein synthesis contribute to the inhibition of cell growth, mostly by inhibition of DNA synthesis. Lymphocytes of CLL when exposed, in vitro, to the compound 2-fluoro-ara-A lead to extensive DNA fragmentation and apoptosis.
References
Ross et al. (1993), A review of its pharmacological properties and therapeutic potential in malignancy; Drugs, 45 737 Gandhi and Plunkett (2002), Cellular and clinical pharmacology of fludarabine; Pharmacokinet. 41 93 Langenhorst et al. (2019), Fludarabine exposure in the conditioning prior to allogeneic hematopoietic cell transplantation predicts outcomes; Blood Adv., 3 2179 Nishioka et al. (2008), Fludarabine induces growth arrest and apoptosis of cytokine- or alloantigen-stimulated peripheral blood mononuclear cells and decreases production of Th1 cytokines via inhibition of nuclear factor kappaB; Bone Marrow Transplant., 41 303 Jensen et al. (2012), Cytotoxic purine nucleoside analogues bind to A1, A2A and A3 adenosine receptors; Naunyn Schmiedebergs Arch. Pharmacol., 385 519
Properties of Fludarabine
| Melting point: | 265-268°C |
| Boiling point: | 747.3±70.0 °C(Predicted) |
| alpha | D25 +17 ±2.5° (c = 0.1 in ethanol) |
| Density | 2.17±0.1 g/cm3(Predicted) |
| storage temp. | 2-8°C |
| solubility | DMF: 20 mg/mL, clear, faintly yellow |
| form | Powder |
| pka | 13.05±0.70(Predicted) |
| color | White to Pale Yellow |
| Water Solubility | Soluble in DMF, DMSO, methanol or ethanol. Sparingly soluble in water |
| Merck | 13,4152 |
| BRN | 1225932 |
| Stability: | Stable for 1 year from date of purchase as supplied. Solutions in DMSO may be stored at -20°C for up to 3 months. |
| InChI | InChI=1/C10H12FN5O4/c11-10-14-7(12)4-8(15-10)16(2-13-4)9-6(19)5(18)3(1-17)20-9/h2-3,5-6,9,17-19H,1H2,(H2,12,14,15)/t3-,5-,6+,9-/s3 |
| CAS DataBase Reference | 21679-14-1(CAS DataBase Reference) |
Safety information for Fludarabine
| Signal word | Warning |
| Pictogram(s) |
 Health Hazard GHS08 |
| GHS Hazard Statements |
H341:Germ cell mutagenicity |
| Precautionary Statement Codes |
P201:Obtain special instructions before use. P202:Do not handle until all safety precautions have been read and understood. P280:Wear protective gloves/protective clothing/eye protection/face protection. P308+P313:IF exposed or concerned: Get medical advice/attention. P405:Store locked up. P501:Dispose of contents/container to..… |
Computed Descriptors for Fludarabine
| InChIKey | HBUBKKRHXORPQB-VOSPVXAENA-N |
| SMILES | C12N=C(N=C(N)C=1N=CN2[C@H]1[C@@H](O)[C@H](O)[C@@H](CO)O1)F |&1:10,11,13,15,r| |
New Products
Methyl (R)-1-Boc-4,4-difluoropyrrolidine-2-carboxylate 2,2-Difluoropropylamine hydrochloride tert-butyl 3-bromoazetidine-1-carboxylate (R)-1-Boc-3-hydroxypyrrolidine DIFLUOROACETIC ANHYDRIDE 2,2-Difluoropropionic acid Diallylamine, 99% Calcium hydroxide, 95% Aluminum oxide, basic 2-Bromophenylacetonitrile, 97% L-tert-Leucine,97% N-Hydroxy-2-methylpropanimidamide 4-(3,4-Dichlorophenyl)-3,4-Dihydro-N-Methyl-1-(2H)-Naphthalenimine (Schiff Base) 2-AMINO-3,5-DIBROMO BENZALDEHYDE [ADBA] L-Glutamic Acid Dimethyl Ester Hcl 10-Methoxy-5H-dibenz[b,f]azepine 5-Cyanophthalide N, N-Carbonyldiimidazole (CDI) Dibenzoyl Peroxide Titanium Dioxide 2-(Methylthio) Benzonitrile Sodium Acetate Anhydrous Allopurinol 1,5-DibromopentaneRelated products of tetrahydrofuran

You may like
-
 21679-14-1 Fludarabine 98%View Details
21679-14-1 Fludarabine 98%View Details
21679-14-1 -
 2-Fluoro-9-beta-D-arabinofuranosyladenine CAS 21679-14-1View Details
2-Fluoro-9-beta-D-arabinofuranosyladenine CAS 21679-14-1View Details
21679-14-1 -
 Fludarabine CAS 21679-14-1View Details
Fludarabine CAS 21679-14-1View Details
21679-14-1 -
 2-Fluoroadenine-9-β-D-arabinofuranoside CAS 21679-14-1View Details
2-Fluoroadenine-9-β-D-arabinofuranoside CAS 21679-14-1View Details
21679-14-1 -
 Ethyl-2-Chloroacetoacetate 609-15-4View Details
Ethyl-2-Chloroacetoacetate 609-15-4View Details
609-15-4 -
 609-15-4View Details
609-15-4View Details
609-15-4 -
![1-(6-Methylpyridin-3-Yl)-2-[4-(Methylsulfonyl)Phenyl]Ethanone [Ketosulfone] 99%](https://img.chemicalbook.in//Content/image/CP5.jpg) 1-(6-Methylpyridin-3-Yl)-2-[4-(Methylsulfonyl)Phenyl]Ethanone [Ketosulfone] 99%View Details
1-(6-Methylpyridin-3-Yl)-2-[4-(Methylsulfonyl)Phenyl]Ethanone [Ketosulfone] 99%View Details
221615-75-4 -
 27143-07-3View Details
27143-07-3View Details
27143-07-3
