Nafcillin sodium salt monohydrate
Synonym(s):6-(2-Ethoxy-1-naphthamido)penicillin;Nafcillin sodium salt monohydrate
- CAS NO.:7177-50-6
- Empirical Formula: C21H25N2NaO6S
- Molecular Weight: 456.49
- MDL number: MFCD01941128
- EINECS: 628-594-2
- SAFETY DATA SHEET (SDS)
- Update Date: 2024-11-19 23:02:33
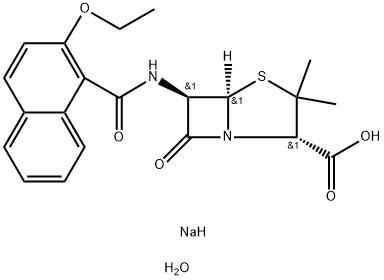
What is Nafcillin sodium salt monohydrate?
Originator
Unipen,Wyeth,US,1964
The Uses of Nafcillin sodium salt monohydrate
Sodium Nafcillin Monohydrate is used as an antibiotic, used in the treatment, used in the treatment of gram-positive bacteria.
The Uses of Nafcillin sodium salt monohydrate
Nafcillin sodium salt monohydrate is an antibiotic resistant to beta-lactamase.
The Uses of Nafcillin sodium salt monohydrate
Antibacterial;Bacterial transpeptidase inhibitor
What are the applications of Application
Nafcillin sodium salt monohydrate is an antibiotic resistant to beta-lactamase
Definition
ChEBI: Nafcillin sodium monohydrate is an organic sodium salt and a hydrate. It contains a nafcillin sodium.
Manufacturing Process
A stirred suspension of 12.6 grams 6-aminopenicillanic acid in 130 ml dry alcohol-free chloroform was treated with 16 ml triethylamine and then with 13.8 grams of a solution of 2-ethoxy-1-naphthoyl chloride in 95 ml chloroform. After being washed successively with 58 ml each of 1 N and then 0.1 N hydrochloric acid the chloroform solution was extracted with N aqueous sodium bicarbonate (58 ml + 6 ml). The combined bicarbonate extracts were washed with 20 ml ether and then evaporated at low temperature and pressure to give the crude sodium salt of 2-ethoxy-1-naphthylpenicillin [also called sodium 6-(2-ethoxy-1-naphthamido)penicillinate] as a yellow powder (20.3 grams). This was dissolved in 20 ml water at 30°C and diluted with 180 ml n-butanol, also at 30°C, with stirring. Slow cooling to 0°C gave colorless needles of the product.
brand name
Nallpen (GlaxoSmithKline).
Therapeutic Function
Antibacterial
Clinical Use
Nafcillin sodium, 6-(2-ethoxy-1-naphthyl)penicillin sodium(Unipen), is another semisynthetic penicillin that resultedfrom the search for penicillinase-resistant compounds. Likemethicillin, nafcillin has substituents in positions ortho tothe point of attachment of the aromatic ring to the carboxamidegroup of penicillin. No doubt, the ethoxy group andthe second ring of the naphthalene group play steric roles instabilizing nafcillin against penicillinase. Very similar structureshave been reported to produce similar results in somesubstituted 2-biphenylpenicillins.
Unlike methicillin, nafcillin is stable enough in acid topermit its use by oral administration. When it is givenorally, its absorption is somewhat slow and incomplete,but satisfactory plasma levels may be achieved in about1 hour. Relatively small amounts are excreted throughthe kidneys; most is excreted in the bile. Even thoughsome cyclic reabsorption from the gut may occur, nafcillingiven orally should be readministered every 4 to 6 hours.This salt is readily soluble in water and may be administeredintramuscularly or intravenously to obtain highplasma concentrations quickly for the treatment of seriousinfections.
Nafcillin sodium may be used in infections causedsolely by penicillin G-resistant staphylococci or whenstreptococci are present also. Although it is recommendedthat it be used exclusively for such resistant infections, nafcillin is also effective against pneumococci and groupA -hemolytic streptococci. Because, like other penicillins,it may cause allergic side effects, it should be administeredwith care.
Properties of Nafcillin sodium salt monohydrate
| storage temp. | 2-8°C |
| solubility | H2O: soluble50mg/mL |
| form | A crystalline solid |
| color | White to off-white |
| CAS DataBase Reference | 7177-50-6(CAS DataBase Reference) |
Safety information for Nafcillin sodium salt monohydrate
| Signal word | Danger |
| Pictogram(s) |
 Exclamation Mark Irritant GHS07  Health Hazard GHS08 |
| GHS Hazard Statements |
H315:Skin corrosion/irritation H317:Sensitisation, Skin H319:Serious eye damage/eye irritation H334:Sensitisation, respiratory H335:Specific target organ toxicity, single exposure;Respiratory tract irritation |
| Precautionary Statement Codes |
P261:Avoid breathing dust/fume/gas/mist/vapours/spray. P280:Wear protective gloves/protective clothing/eye protection/face protection. P284:Wear respiratory protection. P304+P340:IF INHALED: Remove victim to fresh air and Keep at rest in a position comfortable for breathing. P305+P351+P338:IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. Continuerinsing. P342+P311:IF experiencing respiratory symptoms: call a POISON CENTER or doctor/physician. |
Computed Descriptors for Nafcillin sodium salt monohydrate
Nafcillin sodium salt monohydrate manufacturer
JA Sterile Pvt Ltd
New Products
Tert-butyl bis(2-chloroethyl)carbamate (S)-3-Aminobutanenitrile hydrochloride N-Boc-D-alaninol N-BOC-D/L-ALANINOL N-octanoyl benzotriazole 4-Hydrazinobenzoic acid 3,4-Dibenzyloxybenzaldehyde 1,1’-CARBONYLDIIMIDAZOLE R-2-BENZYLOXY PROPIONIC ACID 1,1’-CARBONYLDI (1,2-4 TRIAZOLE) 4-HYDROXY BENZYL ALCOHOL 3-NITRO-2-METHYL ANILINE (2-Hydroxyphenyl)acetonitrile 4-Bromopyrazole 5-BROMO-2CYANO PYRIDINE 5,6-Dimethoxyindanone 5-broMo-2-chloro-N-cyclopentylpyriMidin-4-aMine 4-methoxy-3,5-dinitropyridine 2-(Cyanocyclohexyl)acetic acid 2-aminopropyl benzoate hydrochloride 1-(4-(aminomethyl)benzyl)urea hydrochloride tert-butyl 4- (ureidomethyl)benzylcarbamate diethyl 2-(2-((tertbutoxycarbonyl)amino) ethyl)malonate Ethyl-2-chloro((4-methoxyphenyl)hydrazono)acetateRelated products of tetrahydrofuran




You may like
-
 Nafcillin sodium sterile 98%View Details
Nafcillin sodium sterile 98%View Details -
 Nafcillin sodium salt monohydrate 95.00% CAS 7177-50-6View Details
Nafcillin sodium salt monohydrate 95.00% CAS 7177-50-6View Details
7177-50-6 -
 Nafcillin sodium CAS 7177-50-6View Details
Nafcillin sodium CAS 7177-50-6View Details
7177-50-6 -
 Nafcillin sodium salt monohydrate CAS 7177-50-6View Details
Nafcillin sodium salt monohydrate CAS 7177-50-6View Details
7177-50-6 -
 N-Vinylformamide 99%View Details
N-Vinylformamide 99%View Details
13162-05-5 -
 Chloro Uracil 1820-81-1 99%View Details
Chloro Uracil 1820-81-1 99%View Details
1820-81-1 -
 2-ethyl-6-methyl-3-hydroxypyridine succinate 99%View Details
2-ethyl-6-methyl-3-hydroxypyridine succinate 99%View Details
127464-43-1 -
 2-ETHYLPYRIDINE 100-71-0 99%View Details
2-ETHYLPYRIDINE 100-71-0 99%View Details
100-71-0