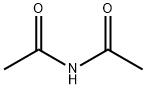
N-Methylacetamide
Synonym(s):Acetylmethylamine
- CAS NO.:79-16-3
- Empirical Formula: C3H7NO
- Molecular Weight: 73.09
- MDL number: MFCD00008683
- EINECS: 201-182-6
- SAFETY DATA SHEET (SDS)
- Update Date: 2025-12-17 09:49:32
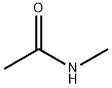
What is N-Methylacetamide?
Chemical properties
colourless liquid or solid. Soluble in water, ethanol, benzene, ether, chloroform, insoluble in petroleum ether.
The Uses of N-Methylacetamide
N-Methylacetamide is used as a chemical intermediate in the production of life science, agrochemicals, electronic materials and construction materials. It is also used as a solvent, in electrochemistry.
Definition
ChEBI: N-methylacetamide is a monocarboxylic acid amide that is the N-methyl derivative of acetamide. It has a role as a metabolite. It is a member of acetamides and a monocarboxylic acid amide. It is functionally related to an acetamide.
Production Methods
Af-Methylacetamide has been prepared by reaction of methylamine with hot acetic acid (D'Alelio and Reid, 1937) and with acetic anhydride (Mauger and Soper, 1946). Other methods include heating iV,N-dimethylurea with acetic acid (US Patent, 1936) and reduction/hydrogenation of N-(hydroxymethyl)acetamide (US Patent, 1944).
Industrial uses
Even though N-methylacetamide shares many general physical and chemical properties with dimethylacetamide, it has not found the extensive industrial applications of the latter. N-methylacetamide dissolves many inorganic salts.
Safety Profile
Moderately toxic by intraperitoneal and subcutaneous routes. Mddly toxic by ingestion and intravenous routes. An experimental teratogen. Experimental reproductive effects. Mutation data reported. When heated to decomposition it emits toxic fumes of NOx.
Metabolism
In a recent comparative toxicity and metabolism study on four formamides and on N-methylacetamide, the sole metabolite of N-methylacetamide in the urine of mice was identified as N-(hydroxymethyl)acetamide (Kestell et al 1987). There was no evidence of induction of hepatic drug metabolizing enzymes in rats following treatment with N-methylacetamide (Ackerman and Leibman, 1977). N-Methylacetamide influenced neither the sleeping time induced by hexobarbital nor the metabolism of hexobarbital or aniline.
Purification Methods
Fractionally distil it under vacuum, then fractionally crystallise it twice from its melt. Likely impurities include acetic acid, methyl amine and H2O. For a detailed purification procedure, see Knecht and Kolthoff, Inorg Chem 1 195 1962. Although N-methylacetamide is commercially available it is often extensively contaminated with acetic acid, methylamine, water and an unidentified impurity. The recommended procedure is to synthesise it in the laboratory by direct reaction. The gaseous amine is passed into hot glacial acetic acid, to give a partially aqueous solution of methylammonium acetate which is heated to ca 130o to expel water. Chemical methods of purification such as extraction by pet ether, treatment with H2SO4, K2CO3 or CaO can be used but are more laborious. Tests for purity include the Karl Fischer titration for water; this can be applied directly. Acetic acid and methylamine can be detected polarographically. In addition to the above, purification of N-methylacetamide can be achieved by fractional freezing, including zone melting, repeated many times, or by vacuum distillation under reduced pressures. For details of zone melting techniques, see Knecht in Recommended Methods for Purification of Solvents and Tests for Impurities, Coetzee Ed. Pergamon Press 1982.[Beilstein 4 IV 176.]
Properties of N-Methylacetamide
| Melting point: | 26-28 °C (lit.) |
| Boiling point: | 204-206 °C (lit.) |
| Density | 0.957 g/mL at 25 °C (lit.) |
| vapor pressure | 12-3680Pa at 15-113℃ |
| refractive index | n |
| Flash point: | 227 °F |
| storage temp. | Inert atmosphere,Room Temperature |
| solubility | miscible with ethanol, ether, acetone, water, chloroform, benzene |
| form | Solid |
| pka | 16.61±0.46(Predicted) |
| color | Colourless Low-Melting |
| PH | 7 (H2O) |
| explosive limit | 3.2-18.1%(V) |
| Water Solubility | soluble |
| BRN | 1071255 |
| Dielectric constant | 178.90000000000001 |
| Stability: | Stable. Combustible. Incompatible with strong oxidizing agents. |
| CAS DataBase Reference | 79-16-3(CAS DataBase Reference) |
| NIST Chemistry Reference | Acetamide, N-methyl-(79-16-3) |
| EPA Substance Registry System | N-Methylacetamide (79-16-3) |
Safety information for N-Methylacetamide
| Signal word | Danger |
| Pictogram(s) |
 Health Hazard GHS08 |
| Precautionary Statement Codes |
P201:Obtain special instructions before use. P202:Do not handle until all safety precautions have been read and understood. P280:Wear protective gloves/protective clothing/eye protection/face protection. P308+P313:IF exposed or concerned: Get medical advice/attention. P405:Store locked up. P501:Dispose of contents/container to..… |
Computed Descriptors for N-Methylacetamide
| InChIKey | OHLUUHNLEMFGTQ-UHFFFAOYSA-N |
N-Methylacetamide manufacturer
New Products
4,4-Difluoropiperidine hydrochloride tert-butyl 9-methoxy-3-azaspiro[5.5]undecane-3-carboxylate Indole Methyl Resin N-Isopropylurea N,N-Dicyclohexylcarbodiimide(DCC) MELDRUMS ACID 5-METHYLISOXAZOLE-4-CARBOXYLIC ACID Magnessium Bis glycinate Zinc ascorbate 1-bromo-2-butyne 2-acetamidophenol 9(10H)-anthracenone Erythrosin B, 4-Piperidinopiperidine 2-((4-morpholinophenylamino) (methylthio) methylene) malononitrile 2,4-dihydroxybenzaldehyde 3-(4-morpholinophenylamino)-5-amino-1H-pyrazole-4-carbonitrile Methyl 2-methylquinoline-6-carboxylate 2,6-dichloro-4-nitropyridine 4-Bromo-2-chlorobenzonitrile 2-(benzylamino)acetic acid hydrochloride 4-(tert-Butoxycarbonylamino)but- 2-ynoic acid 3,4-dihydro-2H-benzo[b][1,4]dioxepine 1-Phenyl-1-cycloprppanecarboxylicacidRelated products of tetrahydrofuran




You may like
-
 79-16-3 99%View Details
79-16-3 99%View Details
79-16-3 -
 N-Methylacetamide CAS 79-16-3View Details
N-Methylacetamide CAS 79-16-3View Details
79-16-3 -
 N-Methylacetamide 97% CAS 79-16-3View Details
N-Methylacetamide 97% CAS 79-16-3View Details
79-16-3 -
 n-Methylacetamide CAS 79-16-3View Details
n-Methylacetamide CAS 79-16-3View Details
79-16-3 -
 N-Methylacetamide CAS 79-16-3View Details
N-Methylacetamide CAS 79-16-3View Details
79-16-3 -
 20677-73-0 (2,2-diethoxyethyl)methylamine 98%View Details
20677-73-0 (2,2-diethoxyethyl)methylamine 98%View Details
20677-73-0 -
 3-(4-(hydroxyamino)-1-oxoisoindolin-2-yl)piperidine-2,6-dione 98%View Details
3-(4-(hydroxyamino)-1-oxoisoindolin-2-yl)piperidine-2,6-dione 98%View Details -
 57381-49-4 2-bromo-4-chlorobenzonitrile 98%View Details
57381-49-4 2-bromo-4-chlorobenzonitrile 98%View Details
57381-49-4
