N-Acetyl-D-Glucosamine
Synonym(s):N-Acetyl-D -glucosamine;D -GlcNAc;2-Acetamido-2-deoxy-D -glucose;N-Acetyl-D-glucosamine - CAS 7512-17-6 - Calbiochem;NAG, GlcNAc
- CAS NO.:7512-17-6
- Empirical Formula: C8H15NO6
- Molecular Weight: 221.21
- MDL number: MFCD00136044
- EINECS: 231-368-2
- SAFETY DATA SHEET (SDS)
- Update Date: 2025-12-26 12:07:08
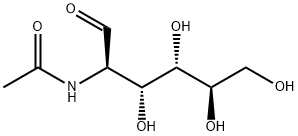
What is N-Acetyl-D-Glucosamine?
Absorption
Approximately 90% of orally administered glucosamine (salt form) gets absorbed from the small intestine.
Toxicity
Mouse, intravenous LD50 is 4170 mg/kg. Side effects that have been reported are mainly mild gastrointestinal complaints such as heartburn, epigastric distress and diarrhea. No allergic reactions have been reported including sulfa-allergic reactions to glucosamine sulfate.
Description
N-Acetyl-D-Glucosamine is the N-acetyl derivative of glucosamine.Chemically it is an amide between glucosamine and acetic acid. A single N-acetlyglucosamine moiety linked to serine or threonine residues on nuclear and cytoplasmic proteins -O-GlcNAc, is an ubiquitous post-translational protein modification. O-GlcNAc modified proteins are involved in sensing the nutrient status of the surrounding cellular environment and adjusting the activity of cellular proteins accordingly. O-GlcNAc regulates cellular responses to hormones such as insulin, initiates a protective response to stress, modulates a cell's capacity to grow and divide, and regulates gene transcription. In humans, it exists in skin, cartilage and blood vessel as a component of hyaluronic acid, and bone tissue, cornea and aorta as a component of keratan sulfate.
N-Acetyl-D-glucosamine (D-GlcNAc) is a derivitized glucose monomer found in polymers of bacterial cell walls, chitin, hyaluronic acids and various glycans. It is also synthesized in the glycosylation pathway as uridine diphosphate (UDP-GlcNAc), which can then be released following degradation of glycosylated proteins.D-GlcNAc is used to identify, differentiate and characterize N-acetyl-β-D-hexoaminidase(s). It is also used in the treatment of osteoarthritis and inflammatory bowel disease (IBD), including ulcerative colitis and Crohn's disease. Further, it is used as a substrate in sialic acid production, cosmetics and in drug development research.
Chemical properties
White powder
The Uses of N-Acetyl-D-Glucosamine
N-acetyl-D-Glucosamine (GlcNAc) is a monosaccharide derivative of glucose. It is released by the action of O-GlcNAcase, in mammalian systems from proteins that have been post-translationally modified with O-GlcNAc. Levels of O-GlcNAcylation proteins from Alzheimer’s disease brain extracts are decreased as compared to that in controls, suggesting that release of GlcNAc may contribute to pathogenesis.1 In E. coli, GlcNAc induces the expression of multidrug exporter genes, indicating that this sugar can alter gene expression.1 GlcNAc is also the monomeric unit of chitin, which is found in fungi and many invertebrates, including crustaceans, insects, and nematodes. For this reason, chemicals that inhibit the incorporation of GlcNAc into chitin are cytotoxic to these organisms.
- Atypical microbial danger signal that acts as a new activator of NLRP3 inflammasome by dissociating the enzyme hexokinase from the mitochondria. D-GlcNAc inhibits purified hexokinase, which is also involved in the glucose metabolism and obesity in mM range. For hexokinase dissociation from the mitochondria much higher concentrations are needed.
- Acceptor substrate for galactosyltransferases. Inhibits the lectin WGA and is used to identify, differentiate and characterize N-acetyl-β-D-hexosaminidase(s).
- Used in the treatment of osteoarthritis and inflammatory bowel disease (IBD), including ulcerative colitis and Crohn's disease. Significantly enhances the prevention of joint damage and inhibits elastase activity and superoxide release from human polymorphonuclear leukocytes. Acts as a cytoprotective agent restoring the integrity and normal function of mucous membrane in humans.
- D-GlcNAc enhances the proliferation and collagen expression of fibroblasts, reduces hyperpigmentation and is therefore considered a valuable ingredient in cosmetics for improving skin wrinkles and color.
- Important substrate for the production of sialic acids.
- Used in multiple other applications in drug development and food supplement, based on a newly described bio-wave model.
The Uses of N-Acetyl-D-Glucosamine
N-Acetyl-D-glucosamine is used in the identification and characterization of N-acetyl-beta-D-hexoaminidase. It is also used in the treatment of osteoarthritis and inflammatory bowel disease (IBD), including ulcerative colitis and Crohn's disease. Further, it is used as a substrate in sialic acid production, cosmetics and in drug development research.
Indications
For the treatment and prevention of osteoarthritis, by itself or in combination with chondroitin sulfate.
Background
The N-acetyl derivative of glucosamine.
What are the applications of Application
N-Acetyl-D-glucosamine is an N-acetyl derivative of glucosamine
Definition
ChEBI: N-acetyl-D-glucosamine is the D isomer of N-acetylglucosamine. It has a role as a bacterial metabolite. It is a N-acetylglucosamine and a N-acetyl-D-hexosamine.
General Description
N-Acetylglucosamine (GlcNAc) is a monosaccharide, derived from glucose and undergoes linear polymerization by (1,4)-β-linkages to form the polymer chitin. It is also a component of heterogenous polysaccharides, such as murein and hyaluronic acid. N-acetylglucosamine exhibits anti-inflammatory properties and is considered as a potential drug in the treatment of osteoarthritis. Its antiarthritic mode of mechanism involves the suppression of IL-1β (Interleuken-1β)-induced NO (nitric oxide), cyclooxygenase-2 (COX-2), and IL-6 production by inhibition of expression of iNOS (inducible nitric oxide synthase) protein and mRNA.
Pharmaceutical secondary standards for application in quality control, provide pharma laboratories and manufacturers with a convenient and cost-effective alternative to the preparation of in-house working standards.
Flammability and Explosibility
Not classified
Biochem/physiol Actions
N-Acetylglucosamine (GlcNAc) oligomer may have the ability to initiate factors for canine polymorphonuclear cells (PMN) in vivo. It possesses wound healing and chemotactic activity. GlcNAc and its derivatives are usually employed in preparing dietary supplements and also used in therapeutic development.
Metabolism
A significant fraction of ingested glucosamine is catabolized by first-pass metabolism in the liver.
Properties of N-Acetyl-D-Glucosamine
| Melting point: | 211 °C (dec.) (lit.) |
| alpha | 42 º (c=2,water,2 hrs) |
| vapor pressure | 8Pa at 20℃ |
| refractive index | +39.0 to +42.0 ° (C=1, H2O) |
| storage temp. | -20°C |
| solubility | Soluble in concentrated hydrochloric acid, sulfuric acid, phosphoric acid and formic acid. Insoluble in water, dilute acids, dilute alkalis, concentrated alkalis and organic solvents. |
| form | saline suspension |
| Boiling point: | 636.4±55.0 °C(Predicted) |
| Density | 1.54 g/cm3 |
| pka | 13.04±0.20(Predicted) |
| color | white to off-white |
| Water Solubility | H2O: 50 mg/mL colorless to faint yellow solution, clear to very slightly hazy |
| Sensitive | Hygroscopic |
| Merck | 14,4458 |
| BRN | 1727589 |
| Stability: | Stable. Incompatible with strong oxidizing agents. |
| CAS DataBase Reference | 7512-17-6(CAS DataBase Reference) |
| EPA Substance Registry System | N-Acetylglucosamine (7512-17-6) |
Safety information for N-Acetyl-D-Glucosamine
| Signal word | Danger |
| Pictogram(s) |
 Corrosion Corrosives GHS05 |
| GHS Hazard Statements |
H314:Skin corrosion/irritation |
| Precautionary Statement Codes |
P280:Wear protective gloves/protective clothing/eye protection/face protection. P310:Immediately call a POISON CENTER or doctor/physician. P305+P351+P338:IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. Continuerinsing. |
Computed Descriptors for N-Acetyl-D-Glucosamine
| InChIKey | OVRNDRQMDRJTHS-ROGOILFBSA-N |
N-Acetyl-D-Glucosamine manufacturer
Siddhi Vinayaka Spechem Private Limited
New Products
4,4-Difluoropiperidine hydrochloride tert-butyl 9-methoxy-3-azaspiro[5.5]undecane-3-carboxylate Indole Methyl Resin N-Isopropylurea N,N-Dicyclohexylcarbodiimide(DCC) MELDRUMS ACID 5-METHYLISOXAZOLE-4-CARBOXYLIC ACID Magnessium Bis glycinate Zinc ascorbate 1-bromo-2-butyne 2-acetamidophenol 9(10H)-anthracenone Erythrosin B, 4-Piperidinopiperidine 2-((4-morpholinophenylamino) (methylthio) methylene) malononitrile 2,4-dihydroxybenzaldehyde 3-(4-morpholinophenylamino)-5-amino-1H-pyrazole-4-carbonitrile Methyl 2-methylquinoline-6-carboxylate 2,6-dichloro-4-nitropyridine 4-Bromo-2-chlorobenzonitrile 2-(benzylamino)acetic acid hydrochloride 4-(tert-Butoxycarbonylamino)but- 2-ynoic acid 3,4-dihydro-2H-benzo[b][1,4]dioxepine 1-Phenyl-1-cycloprppanecarboxylicacidRelated products of tetrahydrofuran

You may like
-
 7512-17-6 98%View Details
7512-17-6 98%View Details
7512-17-6 -
 N-Acetyl-D-glucosamine 96% CAS 7512-17-6View Details
N-Acetyl-D-glucosamine 96% CAS 7512-17-6View Details
7512-17-6 -
 N-Acetyl-D-Glucosamine extrapure AR CAS 7512-17-6View Details
N-Acetyl-D-Glucosamine extrapure AR CAS 7512-17-6View Details
7512-17-6 -
 n-Acetyl-D-glucosamine CAS 7512-17-6View Details
n-Acetyl-D-glucosamine CAS 7512-17-6View Details
7512-17-6 -
 N-Acetyl-D-glucosamine CAS 7512-17-6View Details
N-Acetyl-D-glucosamine CAS 7512-17-6View Details
7512-17-6 -
 Powder N-Acetyl-D-Glucosamine, For Industrial, PrescriptionView Details
Powder N-Acetyl-D-Glucosamine, For Industrial, PrescriptionView Details
7512-17-6 -
 Acetyl Glucosamine PowderView Details
Acetyl Glucosamine PowderView Details
7512-17-6 -
 N Acetyl D Glucosamine, IPView Details
N Acetyl D Glucosamine, IPView Details
7512-17-6
